Changes in the Gut Microbiota of Rats in High-Altitude Hypoxic Environments
- PMID: 36301100
- PMCID: PMC9769726
- DOI: 10.1128/spectrum.01626-22
Changes in the Gut Microbiota of Rats in High-Altitude Hypoxic Environments
Abstract
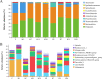
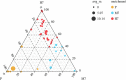
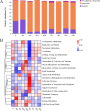
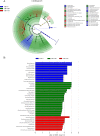
This study was conducted to investigate the effects of high-altitude hypoxic environments on the gut microbiota. Male Sprague-Dawley rats were randomly divided into three groups, namely, the plain, moderate-altitude hypoxic, and high-altitude hypoxic groups. On the 3rd, 7th, 15th, and 30th days of exposure, fecal samples were collected and analyzed via 16S rRNA gene sequencing technology. Fecal microbiota transplantation (FMT) experiments were also performed. The results showed significant differences between the gut microbiota structure and diversity of rats in the high-altitude hypoxic group and those of rats in the other groups. Further, compared with that of rats in the plain group, the gut microbiota of rats in the two hypoxic groups showed the most significant changes on day 7. Furthermore, the gut microbiota of the rats in the FMT groups exhibited changes and became increasingly similar to those of the rats in the hypoxic groups. We also identified the phylum Firmicutes, genus Akkermansia, and genus Lactobacillus as the core microbiota under hypoxic conditions. Phenotypic analysis indicated a decrease in the proportion of aerobic bacteria and an increase in that of anaerobic bacteria, possibly owing to the high-altitude hypoxic environment. Additionally, functional analysis showed significant differences between the different groups with respect to different metabolic pathways, including carbohydrate metabolism, energy metabolism, glycan biosynthesis, and metabolism. These findings indicated significant changes in gut microbiota structure and diversity under high-altitude hypoxia, establishing a foundation for further research on the pathogenesis and development of diseases, as well as drug metabolism, under high-altitude hypoxia. IMPORTANCE In this study, we investigated the effects of high-altitude hypoxic environments with low oxygen levels on the gut microbiota characteristics of rats. We observed that high-altitude hypoxia is an important environmental factor that can affect gut microbiota structure and diversity, thereby affecting homeostasis in the host intestinal environment. These findings provide a basis for further studies on disease initiation and development, as well as drug metabolism, in high-altitude hypoxic environments.
Keywords: 16S rRNA gene; diversity; fecal microbiota transplantation; gut microbiota; high-altitude hypoxia; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Altitude-adaption of gut microbiota in Tibetan chicken.Poult Sci. 2022 Sep;101(9):101998. doi: 10.1016/j.psj.2022.101998. Epub 2022 Jun 10. Poult Sci. 2022. PMID: 35841636 Free PMC article.
-
Human gut microbiota adaptation to high-altitude exposure: longitudinal analysis over acute and prolonged periods.Microbiol Spectr. 2025 Jun 3;13(6):e0291624. doi: 10.1128/spectrum.02916-24. Epub 2025 Apr 21. Microbiol Spectr. 2025. PMID: 40257273 Free PMC article.
-
Acute exposure to simulated high-altitude hypoxia alters gut microbiota in mice.Arch Microbiol. 2022 Jun 22;204(7):412. doi: 10.1007/s00203-022-03031-4. Arch Microbiol. 2022. PMID: 35731330
-
Gut Microbiota as the Potential Mechanism to Mediate Drug Metabolism Under High-altitude Hypoxia.Curr Drug Metab. 2022;23(1):8-20. doi: 10.2174/1389200223666220128141038. Curr Drug Metab. 2022. PMID: 35088664 Review.
-
Effects of Gut Microbiota on Drug Metabolism and Guidance for Rational Drug Use Under Hypoxic Conditions at High Altitudes.Curr Drug Metab. 2019;20(2):155-165. doi: 10.2174/1389200219666181019145159. Curr Drug Metab. 2019. PMID: 30338735 Review.
Cited by
-
Multi-omics insights into the energy compensation of rumen microbiota of grazing yaks in cold season.Front Microbiol. 2024 Oct 9;15:1467841. doi: 10.3389/fmicb.2024.1467841. eCollection 2024. Front Microbiol. 2024. PMID: 39444681 Free PMC article.
-
Intestinal oxygen utilisation and cellular adaptation during intestinal ischaemia-reperfusion injury.Clin Transl Med. 2025 Jan;15(1):e70136. doi: 10.1002/ctm2.70136. Clin Transl Med. 2025. PMID: 39724463 Free PMC article. Review.
-
Dysregulation of metabolites and high-altitude illnesses development under plateau conditions.Front Physiol. 2025 Jul 2;16:1600374. doi: 10.3389/fphys.2025.1600374. eCollection 2025. Front Physiol. 2025. PMID: 40671709 Free PMC article. Review.
-
Effects of high-/low-temperature and high-altitude hypoxic environments on gut microbiota of sports people: A retrospective analysis.Sports Med Health Sci. 2023 Mar 17;5(2):83-90. doi: 10.1016/j.smhs.2023.03.003. eCollection 2023 Jun. Sports Med Health Sci. 2023. PMID: 37424532 Free PMC article. Review.
-
Brassica rapa L. polysaccharide mitigates hypobaric hypoxia-induced oxidation and intestinal damage via microbiome modulation.NPJ Sci Food. 2024 Dec 27;8(1):112. doi: 10.1038/s41538-024-00365-9. NPJ Sci Food. 2024. PMID: 39730362 Free PMC article.
References
-
- Li XY, Liu YN, Li YP, Yuan M, Zhu JB. 2011. Pharmacokinetic study of sulfamethoxazole in healthy Han volunteers living at plain and native Han and Tibetan healthy volunteers living at high altitude. Yao Xue Xue Bao 46:1117–1122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

