Hypoxia-Inducible Factor 2 Alpha (HIF2α) Inhibitors: Targeting Genetically Driven Tumor Hypoxia
- PMID: 36301191
- PMCID: PMC10216878
- DOI: 10.1210/endrev/bnac025
Hypoxia-Inducible Factor 2 Alpha (HIF2α) Inhibitors: Targeting Genetically Driven Tumor Hypoxia
Abstract
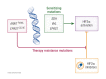
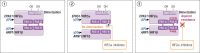
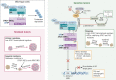
Tumors driven by deficiency of the VHL gene product, which is involved in degradation of the hypoxia-inducible factor subunit 2 alpha (HIF2α), are natural candidates for targeted inhibition of this pathway. Belzutifan, a highly specific and well-tolerated HIF2α inhibitor, recently received FDA approval for the treatment of nonmetastatic renal cell carcinomas, pancreatic neuroendocrine tumors, and central nervous system hemangioblastomas from patients with von Hippel-Lindau disease, who carry VHL germline mutations. Such approval is a milestone in oncology; however, the full potential, and limitations, of HIF2α inhibition in the clinic are just starting to be explored. Here we briefly recapitulate the molecular rationale for HIF2α blockade in tumors and review available preclinical and clinical data, elaborating on mutations that might be particularly sensitive to this approach. We also outline some emerging mechanisms of intrinsic and acquired resistance to HIF2α inhibitors, including acquired mutations of the gatekeeper pocket of HIF2α and its interacting partner ARNT. Lastly, we propose that the high efficacy of belzutifan observed in tumors with genetically driven hypoxia caused by VHL mutations suggests that a focus on other mutations that similarly lead to HIF2α stabilization, such as those occurring in neuroendocrine tumors with disruptions in the tricarboxylic acid cycle (SDHA/B/C/D, FH, MDH2, IDH2), HIF hydroxylases (EGLN/PHDs), and the HIF2α-encoding gene, EPAS1, are warranted.
Keywords: HIF2; VHL; belzutifan; cancer; hypoxia; hypoxia-inducible factor; inhibitor; mutation; neuroendocrine tumor; paraganglioma; pheochromocytoma; pseudohypoxia; resistance; sensitivity; targeted therapy.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of Interests R.A.T. nothing to declare; C.J. receives research support from Lantheus Pharmaceuticals, Progenics, Exelixis, Merck Sharp and Dohme, and Pfizer, and is on the advisory board of Lantheus Pharmaceuticals, Progenics, Merck Sharp and Dohme, Pfizer, and HRA Pharma; G.A.P.: nothing to declare; C.A.: nothing to declare; J.C. receives scientific consultancy (speaker and advisory roles) fees from Novartis, Pfizer, Ipsen, Exelixis, Bayer, Eisai, Advanced Accelerator Applications, Amgen, Sanofi, Lilly, Hudchinson Pharma, ITM, Advanz, Merck Serono, and Esteve, and receives research support from Novartis, Pfizer, Astrazeneca, Advanced Accelerator Applications, Eisai, Amgen, and Bayer, and research grants from Novartis, Pfizer, Astrazeneca, Advanced Accelerator Applications, Eisai, Amgen, and Bayer; P.L.M.D.: nothing to declare.
Figures
References
-
- Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2(12):758–770. - PubMed
-
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56(19):4509–4515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

