Extrapolated longer-term effects of the DAPA-CKD trial: a modelling analysis
- PMID: 36301617
- PMCID: PMC10157747
- DOI: 10.1093/ndt/gfac280
Extrapolated longer-term effects of the DAPA-CKD trial: a modelling analysis
Abstract
Background: The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial assessed dapagliflozin versus placebo, in addition to standard therapy, in patients with chronic kidney disease (CKD) and albuminuria, and was terminated prematurely due to overwhelming efficacy. The study objective was to model the long-term clinical outcomes of DAPA-CKD beyond the trial follow-up.
Methods: A Markov model extrapolated event incidence per 1000 patients and CKD progression rates for patients receiving dapagliflozin or placebo over a 10-year time horizon. We derived treatment-specific CKD stage transition matrices using DAPA-CKD trial data. We extrapolated relevant efficacy endpoints using parametric survival equations for all-cause mortality and generalized estimating equations for recurrent events.
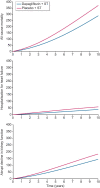
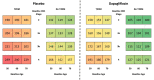
Results: When extrapolated over a 10-year period, patients randomized to dapagliflozin spent more time in CKD stages 1-3 and less in stages 4-5 than placebo [0.65 (95% CrI 0.41, 0.90) and -0.23 (95% CrI -0.45, 0.00) years per patient, respectively]. Dapagliflozin prevented an estimated 83 deaths and 51 patients initiating kidney replacement therapy per 1000 patients over 10 years. Predicted rates of hospitalized heart failure and abrupt declines in kidney function were reduced (19 and 39 estimated events per 1000 patients, respectively).
Conclusions: Adding dapagliflozin to standard therapeutic management of CKD is expected to have long-term cardiorenal benefit beyond what has been demonstrated in the DAPA-CKD trial, with patients predicted to live longer with fewer complications.
Keywords: SGLT2 inhibitor; chronic kidney disease; dapagliflozin; dialysis; kidney transplantation.
© The Author(s) 2022. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
P.M. and R.B. are/were employees of Health Economics and Outcomes Research Ltd, Cardiff, UK. Health Economics and Outcomes Research Ltd received fees from AstraZeneca in relation to this study. J.J.G.S., C.D.S., B.S. and S.N. are employees of AstraZeneca. R.C.-R. has received honoraria as consultant from: AstraZeneca, Boehringer Ingelheim, Janssen, Bayer, Chinook, AbbVie and Novo Nordisk, and research support from AstraZeneca, GSK and Novo Nordisk. P.R. has received honoraria to Steno Diabetes Center Copenhagen for consultancy from AstraZeneca, Astellas, Bayer, Boehringer Ingelheim, Gilead, Novo Nordisk, Merck, Mundipharma, Sanofi and Vifor, and research support from AstraZeneca and Novo Nordisk. G.M.C. has received honoraria as consultant from: Akebia, Amgen, Ardelyx, AstraZeneca, Baxter, Cricket, DiaMedica, Gilead, Miromatrix, Outset, Reata, Sanifit and Vertex. He has received research support from Amgen and Janssen. He has served on data and safety monitoring boards for Angion, Bayer and Recor. J.J.V.M. reports non-financial support and other from AstraZeneca, during the conduct of the study; non-financial support and other from Cardiorentis, Amgen, Oxford University/Bayer, Theracos, AbbVie, other from DalCor, Pfizer, Novartis, GSK, Vifor-Fresenius, Kidney Research UK, Bayer, Merck and Bristol-Myers Squibb, outside the submitted work. D.C.W. provides ongoing consultancy services to AstraZeneca and has received honoraria and/or consultancy fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bayer, GSK, Janssen, Napp, Mundipharma, Medscape, Merck Sharp and Dohme, Pharmacosmos, Reata, Takeda and Vifor-Fresenius. H.J.L.H. is a consultant for AstraZeneca, AbbVie, Boehringer Ingelheim, CSL Behring, Bayer, Chinook, Dimerix, Gilead, Goldfinch, Merck, Novo Nordisk, Janssen and Travere Pharmaceuticals. He received research support from AstraZeneca, Boehringer Ingelheim, Janssen and Novo Nordisk.
Figures





Similar articles
-
The long-term effects of dapagliflozin in chronic kidney disease: a time-to-event analysis.Nephrol Dial Transplant. 2024 Nov 27;39(12):2040-2047. doi: 10.1093/ndt/gfae106. Nephrol Dial Transplant. 2024. PMID: 38730538 Free PMC article. Clinical Trial.
-
Translating the efficacy of dapagliflozin in chronic kidney disease to lower healthcare resource utilization and costs: a medical care cost offset analysis.J Med Econ. 2023 Jan-Dec;26(1):1407-1416. doi: 10.1080/13696998.2023.2264715. Epub 2023 Oct 31. J Med Econ. 2023. PMID: 37807895 Review.
-
Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial.Lancet Diabetes Endocrinol. 2021 Nov;9(11):755-766. doi: 10.1016/S2213-8587(21)00243-6. Epub 2021 Oct 4. Lancet Diabetes Endocrinol. 2021. PMID: 34619106 Clinical Trial.
-
The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics.Nephrol Dial Transplant. 2020 Oct 1;35(10):1700-1711. doi: 10.1093/ndt/gfaa234. Nephrol Dial Transplant. 2020. PMID: 32862232 Free PMC article. Clinical Trial.
-
Sodium-Glucose Cotransporter 2 Inhibitors in Patients with Non-Diabetic Chronic Kidney Disease.Adv Ther. 2021 May;38(5):2201-2212. doi: 10.1007/s12325-021-01735-5. Epub 2021 Apr 16. Adv Ther. 2021. PMID: 33860925 Review.
Cited by
-
Mind the Gap in Kidney Care: Translating What We Know into What We Do.Indian J Nephrol. 2024 Jul-Aug;34(4):281-290. doi: 10.25259/IJN_145_2024. Epub 2024 Jun 29. Indian J Nephrol. 2024. PMID: 39156847 Free PMC article. No abstract available.
-
The Future of Chronic Kidney Disease Treatment: Combination Therapy (Polypill) or Biomarker-Guided Personalized Intervention?Biomolecules. 2025 Jun 3;15(6):809. doi: 10.3390/biom15060809. Biomolecules. 2025. PMID: 40563449 Free PMC article. Review.
-
Mind the Gap in Kidney Care: Translating What We Know Into What We Do.Am J Hypertens. 2024 Jul 15;37(8):640-649. doi: 10.1093/ajh/hpae056. Am J Hypertens. 2024. PMID: 39004933 Free PMC article. No abstract available.
-
Dapagliflozin in chronic kidney disease: cost-effectiveness beyond the DAPA-CKD trial.Clin Kidney J. 2024 Feb 9;17(2):sfae025. doi: 10.1093/ckj/sfae025. eCollection 2024 Feb. Clin Kidney J. 2024. PMID: 38389710 Free PMC article.
-
Sodium-Glucose Co-Transporter 2 Inhibitors: Mechanism of Action and Efficacy in Non-Diabetic Kidney Disease from Bench to Bed-Side.J Clin Med. 2024 Feb 7;13(4):956. doi: 10.3390/jcm13040956. J Clin Med. 2024. PMID: 38398269 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

