Species-specific differences in NPC1 protein trafficking govern therapeutic response in Niemann-Pick type C disease
- PMID: 36301667
- PMCID: PMC9746915
- DOI: 10.1172/jci.insight.160308
Species-specific differences in NPC1 protein trafficking govern therapeutic response in Niemann-Pick type C disease
Abstract
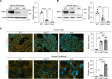
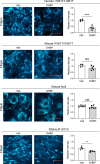
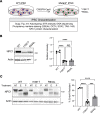
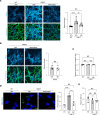
The folding and trafficking of transmembrane glycoproteins are essential for cellular homeostasis and are compromised in many diseases. In Niemann-Pick type C disease, a lysosomal disorder characterized by impaired intracellular cholesterol trafficking, the transmembrane glycoprotein NPC1 misfolds due to disease-causing missense mutations. While mutant NPC1 has emerged as a robust target for proteostasis modulators, drug development efforts have been unsuccessful in mouse models. Here, we demonstrated unexpected differences in trafficking through the medial Golgi between mouse and human I1061T-NPC1, a common disease-causing mutant. We established that these distinctions are governed by differences in the NPC1 protein sequence rather than by variations in the endoplasmic reticulum-folding environment. Moreover, we demonstrated direct effects of mutant protein trafficking on the response to small molecules that modulate the endoplasmic reticulum-folding environment by affecting Ca++ concentration. Finally, we developed a panel of isogenic human NPC1 iNeurons expressing WT, I1061T-, and R934L-NPC1 and demonstrated their utility in testing these candidate therapeutics. Our findings identify important rules governing mutant NPC1's response to proteostatic modulators and highlight the importance of species- and mutation-specific responses for therapy development.
Keywords: Lysosomes; Neuroscience; Protein misfolding; Protein traffic; Therapeutics.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

