Mitochondrial dysfunction reactivates α-fetoprotein expression that drives copper-dependent immunosuppression in mitochondrial disease models
- PMID: 36301669
- PMCID: PMC9797342
- DOI: 10.1172/JCI154684
Mitochondrial dysfunction reactivates α-fetoprotein expression that drives copper-dependent immunosuppression in mitochondrial disease models
Abstract
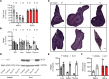
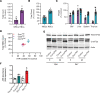
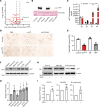
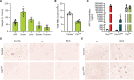
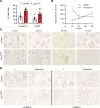
Signaling circuits crucial to systemic physiology are widespread, yet uncovering their molecular underpinnings remains a barrier to understanding the etiology of many metabolic disorders. Here, we identified a copper-linked signaling circuit activated by disruption of mitochondrial function in the murine liver or heart that resulted in atrophy of the spleen and thymus and caused a peripheral white blood cell deficiency. We demonstrated that the leukopenia was caused by α-fetoprotein, which required copper and the cell surface receptor CCR5 to promote white blood cell death. We further showed that α-fetoprotein expression was upregulated in several cell types upon inhibition of oxidative phosphorylation. Collectively, our data argue that α-fetoprotein may be secreted by bioenergetically stressed tissue to suppress the immune system, an effect that may explain the recurrent or chronic infections that are observed in a subset of mitochondrial diseases or in other disorders with secondary mitochondrial dysfunction.
Keywords: Metabolism; Mitochondria.
Conflict of interest statement
Figures
Similar articles
-
Tumor-Derived α-Fetoprotein Suppresses Fatty Acid Metabolism and Oxidative Phosphorylation in Dendritic Cells.Cancer Immunol Res. 2019 Jun;7(6):1001-1012. doi: 10.1158/2326-6066.CIR-18-0513. Epub 2019 Apr 15. Cancer Immunol Res. 2019. PMID: 30988028
-
Copper(II)-binding ability of human alpha-fetoprotein.Cancer Res. 1978 Oct;38(10):3483-6. Cancer Res. 1978. PMID: 80265
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
The complex crosstalk between mitochondria and the nucleus: What goes in between?Int J Biochem Cell Biol. 2015 Jun;63:10-5. doi: 10.1016/j.biocel.2015.01.026. Epub 2015 Feb 7. Int J Biochem Cell Biol. 2015. PMID: 25666554 Review.
-
Multisystem manifestations of mitochondrial disorders.J Neurol. 2009 May;256(5):693-710. doi: 10.1007/s00415-009-5028-3. Epub 2009 Mar 1. J Neurol. 2009. PMID: 19252802 Review.
Cited by
-
Rethinking carnitine palmitoyltransferase II and liver stem cells in metabolic dysfunction-associated fatty liver disease-related hepatocellular carcinoma.World J Gastroenterol. 2025 Apr 21;31(15):104528. doi: 10.3748/wjg.v31.i15.104528. World J Gastroenterol. 2025. PMID: 40309230 Free PMC article.
-
APOE expression and secretion are modulated by mitochondrial dysfunction.Elife. 2023 May 12;12:e85779. doi: 10.7554/eLife.85779. Elife. 2023. PMID: 37171075 Free PMC article.
-
Lymphopoiesis is attenuated upon hepatocyte-specific deletion of the cytochrome c oxidase assembly factor Sco1.iScience. 2025 Mar 3;28(4):112151. doi: 10.1016/j.isci.2025.112151. eCollection 2025 Apr 18. iScience. 2025. PMID: 40177634 Free PMC article.
-
Targeting fatty acid oxidation enhances response to HER2-targeted therapy.Nat Commun. 2024 Aug 3;15(1):6587. doi: 10.1038/s41467-024-50998-3. Nat Commun. 2024. PMID: 39097623 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

