Receipt of First and Second Doses of JYNNEOS Vaccine for Prevention of Monkeypox - United States, May 22-October 10, 2022
- PMID: 36301741
- PMCID: PMC9620573
- DOI: 10.15585/mmwr.mm7143e2
Receipt of First and Second Doses of JYNNEOS Vaccine for Prevention of Monkeypox - United States, May 22-October 10, 2022
Abstract
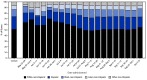
Vaccination with JYNNEOS vaccine (Modified Vaccinia Ankara vaccine, Bavarian Nordic) to prevent monkeypox commenced shortly after confirmation of the first monkeypox case in the current outbreak in the United States on May 17, 2022 (1). To date, more than 27,000 cases have been reported across all 50 states, the District of Columbia (DC), and Puerto Rico.* JYNNEOS vaccine is licensed by the Food and Drug Administration (FDA) as a 0.5-mL 2-dose series administered subcutaneously 28 days apart to prevent smallpox and monkeypox infections (2) and has been found to provide protection against monkeypox infection during the current outbreak (3). The U.S. Department of Health and Human Services (HHS) allocated 1.1 million vials of JYNNEOS vaccine from the Strategic National Stockpile, with doses allocated to jurisdictions based on case counts and estimated size of population at risk (4). However, initial vaccine supplies were severely constrained relative to vaccine demand during the expanding outbreak. Some jurisdictions with highest incidence responded by prioritizing first dose administration during May-July (5,6). The FDA emergency use authorization (EUA) of 0.1 mL dosing for intradermal administration of JYNNEOS for persons aged ≥18 years on August 9, 2022, substantially expanded available vaccine supply† (7). The U.S. vaccination strategy focuses primarily on persons with known or presumed exposures to monkeypox (8) or those at high risk for occupational exposure (9). Data on monkeypox vaccine doses administered and reported to CDC by U.S. jurisdictions were analyzed to assess vaccine administration and completion of the 2-dose series. A total of 931,155 doses of JYNNEOS vaccine were administered and reported to the CDC by 55 U.S. jurisdictions during May 22-October 10, 2022. Among persons who received ≥1 dose, 51.4% were non-Hispanic White (White), 22.5% were Hispanic or Latino (Hispanic), and 12.6% were non-Hispanic Black or African American (Black). The percentages of vaccine recipients who were Black (5.6%) and Hispanic (15.5%) during May 22-June 25 increased to 13.3% and 22.7%, respectively, during July 31-October 10. Among 496,888 persons who received a first dose and were eligible for a second dose during the study period, 57.6% received their second dose. Second dose receipt was highest among older adults, White persons, and those residing in the South U.S. Census Bureau Region. Tracking and addressing disparities in vaccination can reduce inequities, and equitable access to and acceptance of vaccine should be an essential factor in planning vaccination programs, events, and strategies. Receipt of both first and second doses is necessary for optimal protection against Monkeypox virus infection.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Nathaniel Smith reports support from the Association of State and Territorial Health Officials for travel to meetings while serving in an unpaid position as President (September 2019–July 2020). No other potential conflicts of interest were disclosed.
Figures
References
-
- Food and Drug Administration. JYNNEOS [package insert, revised June 2021]. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. https://www.fda.gov/media/131078/download
-
- Administration for Strategic Preparedness and Response. JYNNEOS vaccine distribution by jurisdiction. Washington, DC: US Department of Health and Human Services, Administration for Strategic Preparedness and Response ; 2022. Accessed September 30, 2022. https://aspr.hhs.gov/SNS/Pages/JYNNEOS-Distribution.aspx
-
- City of Philadelphia Department of Health. Monkeypox vaccine update: expanded eligibility & second doses. Philadelphia, PA: City of Philadelphia Department of Health; 2022. https://vax.phila.gov/index.php/notices/monkeypox-vaccine-update-expande...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

