Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in people living with HIV in the Netherlands: A nationwide prospective cohort study
- PMID: 36301821
- PMCID: PMC9612532
- DOI: 10.1371/journal.pmed.1003979
Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in people living with HIV in the Netherlands: A nationwide prospective cohort study
Erratum in
-
Correction: Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in people living with HIV in the Netherlands: A nationwide prospective cohort study.PLoS Med. 2023 Jan 6;20(1):e1004159. doi: 10.1371/journal.pmed.1004159. eCollection 2023 Jan. PLoS Med. 2023. PMID: 36608645 Free PMC article.
Abstract
Background: Vaccines can be less immunogenic in people living with HIV (PLWH), but for SARS-CoV-2 vaccinations this is unknown. In this study we set out to investigate, for the vaccines currently approved in the Netherlands, the immunogenicity and reactogenicity of SARS-CoV-2 vaccinations in PLWH.
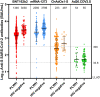
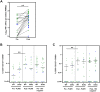
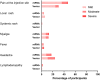
Methods and findings: We conducted a prospective cohort study to examine the immunogenicity of BNT162b2, mRNA-1273, ChAdOx1-S, and Ad26.COV2.S vaccines in adult PLWH without prior COVID-19, and compared to HIV-negative controls. The primary endpoint was the anti-spike SARS-CoV-2 IgG response after mRNA vaccination. Secondary endpoints included the serological response after vector vaccination, anti-SARS-CoV-2 T-cell response, and reactogenicity. Between 14 February and 7 September 2021, 1,154 PLWH (median age 53 [IQR 44-60] years, 85.5% male) and 440 controls (median age 43 [IQR 33-53] years, 28.6% male) were included in the final analysis. Of the PLWH, 884 received BNT162b2, 100 received mRNA-1273, 150 received ChAdOx1-S, and 20 received Ad26.COV2.S. In the group of PLWH, 99% were on antiretroviral therapy, 97.7% were virally suppressed, and the median CD4+ T-cell count was 710 cells/μL (IQR 520-913). Of the controls, 247 received mRNA-1273, 94 received BNT162b2, 26 received ChAdOx1-S, and 73 received Ad26.COV2.S. After mRNA vaccination, geometric mean antibody concentration was 1,418 BAU/mL in PLWH (95% CI 1322-1523), and after adjustment for age, sex, and vaccine type, HIV status remained associated with a decreased response (0.607, 95% CI 0.508-0.725, p < 0.001). All controls receiving an mRNA vaccine had an adequate response, defined as >300 BAU/mL, whilst in PLWH this response rate was 93.6%. In PLWH vaccinated with mRNA-based vaccines, higher antibody responses were predicted by CD4+ T-cell count 250-500 cells/μL (2.845, 95% CI 1.876-4.314, p < 0.001) or >500 cells/μL (2.936, 95% CI 1.961-4.394, p < 0.001), whilst a viral load > 50 copies/mL was associated with a reduced response (0.454, 95% CI 0.286-0.720, p = 0.001). Increased IFN-γ, CD4+ T-cell, and CD8+ T-cell responses were observed after stimulation with SARS-CoV-2 spike peptides in ELISpot and activation-induced marker assays, comparable to controls. Reactogenicity was generally mild, without vaccine-related serious adverse events. Due to the control of vaccine provision by the Dutch National Institute for Public Health and the Environment, there were some differences between vaccine groups in the age, sex, and CD4+ T-cell counts of recipients.
Conclusions: After vaccination with BNT162b2 or mRNA-1273, anti-spike SARS-CoV-2 antibody levels were reduced in PLWH compared to HIV-negative controls. To reach and maintain the same serological responses as HIV-negative controls, additional vaccinations are probably required.
Trial registration: The trial was registered in the Netherlands Trial Register (NL9214). https://www.trialregister.nl/trial/9214.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: All authors have completed the ICMJE disclosure form and declare no competing interests exist directly related to the submitted work Conflicts of interest outside the submitted work CR has received research grants from ViiV, Gilead, ZonMW, AIDSfonds, Erasmus MC, and Health~Holland and honorariums for advisory boards from Gilead and ViiV; WFWB declares reimbursement for participation of patient in trial by GSK to institution. DG and RDdV are supported by the Health~Holland grant EMCLHS20017 co-funded by the PPP Allowance made available by the Health~Holland, Top Sector Life Sciences & Health, to stimulate public–private partnerships. RDdV is listed as inventor of the fusion inhibitory lipopeptide [SARSHRC-PEG4]2-chol on a provisional patent application. VASHD has received research grants from ZonMw, Horizon 2020 – Marie Curie-Sklodowska, Takeda and payments for lectures and advisory boards from Takeda, CSL Behring, Pharming and GSK. KCES received honorariums for advisory boards from Gilead and ViiV. BJAR declares research grants from Gilead and MSD and honorary for advisory boards for Astra Zeneca, Roche, Gilead, F2G all outside the context of this work. RvM received consultancies fees paid to their institution from ViiV; Gilead; MSD, received research grants paid to their institution from ViiV; Gilead All other authors declare hat no competing interests exist.
Figures
References
-
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
-
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–93. doi: 10.1016/S0140-6736(20)32466-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

