Methods and Limitations of Augmenting Mesenchymal Stem Cells for Therapeutic Applications
- PMID: 36301919
- PMCID: PMC10254976
- DOI: 10.1089/wound.2022.0107
Methods and Limitations of Augmenting Mesenchymal Stem Cells for Therapeutic Applications
Abstract
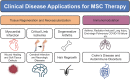
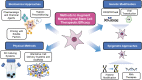
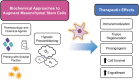
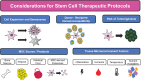
Significance: Given their capacity for self-renewal, multilineage differentiation, and immunomodulatory potential, mesenchymal stem cells (MSCs) represent a promising modality of clinical therapy for both regenerative medicine and immune diseases. In this study, we review the key approaches and popular methods utilized to boost potency and modify functions of MSCs for clinical purposes as well as their associated limitations. Recent Advances: Several major domains of cell modification strategies are currently employed by investigators to overcome these deficits and augment the therapeutic potential of MSCs. Priming MSCs with soluble factors or pharmacologic agents as well as manipulating oxygen availability in culture have been demonstrated to be effective biochemical methods to augment MSC potential. Distinct genetic and epigenetic methods have emerged in recent years to modify the genetic expression of target proteins and factors thereby modulating MSCs capacity for differentiation, migration, and proliferation. Physical methods utilizing three-dimensional culture methods and alternative cell delivery systems and scaffolds can be used to recapitulate the native MSC niche and augment their engraftment and viability for in vivo models. Critical Issues: Unmodified MSCs have demonstrated only modest benefits in many preclinical and clinical studies due to issues with cell engraftment, viability, heterogeneity, and immunocompatibility between donor and recipient. Furthermore, unmodified MSCs can have low inherent therapeutic potential for which intensive research over the past few decades has been dedicated to improving cell functionality and potency.
Keywords: cell-based therapy; mesenchymal stem cell; stem cell therapy.
Conflict of interest statement
This article is supported by grants from the National Institutes of Health R01 HL149452 and HL156152-01A1 Catalyze.
Figures








Similar articles
-
Priming strategies for controlling stem cell fate: Applications and challenges in dental tissue regeneration.World J Stem Cells. 2021 Nov 26;13(11):1625-1646. doi: 10.4252/wjsc.v13.i11.1625. World J Stem Cells. 2021. PMID: 34909115 Free PMC article. Review.
-
Autophagy: a promising therapeutic target for improving mesenchymal stem cell biological functions.Mol Cell Biochem. 2021 Feb;476(2):1135-1149. doi: 10.1007/s11010-020-03978-2. Epub 2020 Nov 16. Mol Cell Biochem. 2021. PMID: 33196943 Review.
-
Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: current status and future prospects.Stem Cell Res Ther. 2022 Apr 4;13(1):146. doi: 10.1186/s13287-022-02822-2. Stem Cell Res Ther. 2022. PMID: 35379361 Free PMC article. Review.
-
Characterization and therapeutic applications of mesenchymal stem cells for regenerative medicine.Tissue Cell. 2020 Jun;64:101330. doi: 10.1016/j.tice.2020.101330. Epub 2020 Jan 10. Tissue Cell. 2020. PMID: 32473704 Review.
-
Influence of 3D printed porous architecture on mesenchymal stem cell enrichment and differentiation.Acta Biomater. 2016 Mar 1;32:161-169. doi: 10.1016/j.actbio.2016.01.007. Epub 2016 Jan 7. Acta Biomater. 2016. PMID: 26773464
Cited by
-
A review on mesenchymal stem cells and their secretome in hepatocellular carcinogenesis and its related signaling pathways: recent update.Discov Oncol. 2025 Jul 7;16(1):1276. doi: 10.1007/s12672-025-03088-9. Discov Oncol. 2025. PMID: 40622501 Free PMC article. Review.
-
Directing cell delivery to murine atherosclerotic aortic lesions via targeting inflamed circulatory interface using nanocarriers.Front Cardiovasc Med. 2025 Jun 24;12:1517320. doi: 10.3389/fcvm.2025.1517320. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40630903 Free PMC article.
-
Epigenetic mechanisms in stem cell therapies for achilles tendinopathy.Front Cell Dev Biol. 2025 Mar 13;13:1516250. doi: 10.3389/fcell.2025.1516250. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40181824 Free PMC article. Review.
-
Mesenchymal stem cell-based therapy for non-healing wounds due to chronic limb-threatening ischemia: A review of preclinical and clinical studies.Front Cardiovasc Med. 2023 Feb 1;10:1113982. doi: 10.3389/fcvm.2023.1113982. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36818343 Free PMC article. Review.
-
Targeted cell delivery of mesenchymal stem cell therapy for cardiovascular disease applications: a review of preclinical advancements.Front Cardiovasc Med. 2023 Aug 4;10:1236345. doi: 10.3389/fcvm.2023.1236345. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37600026 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

