Chicken or Egg? Mitochondrial Phospholipids and Oxidative Stress in Disuse-Induced Skeletal Muscle Atrophy
- PMID: 36301935
- PMCID: PMC9986029
- DOI: 10.1089/ars.2022.0151
Chicken or Egg? Mitochondrial Phospholipids and Oxidative Stress in Disuse-Induced Skeletal Muscle Atrophy
Abstract
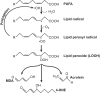
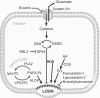
Significance: Accumulation of reactive oxygen species (ROS) is known to promote cellular damage in multiple cell types. In skeletal muscle, ROS has been implicated in disuse-induced muscle atrophy. However, the molecular origin and mechanism of how disuse promotes ROS and muscle dysfunction remains unclear. Recent Advances: Recently, we implicated membrane lipids of mitochondria to be a potential source of ROS to promote muscle atrophy. Critical Issues: In this review, we discuss evidence that changes in mitochondrial lipids represent a physiologically relevant process by which disuse promotes mitochondrial electron leak and oxidative stress. Future Directions: We further discuss lipid hydroperoxides as a potential downstream mediator of ROS to induce muscle atrophy. Antioxid. Redox Signal. 38, 338-351.
Keywords: ROS; disuse; lipid peroxidation; mitochondrial phospholipids; myopathy.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Lipid hydroperoxides promote sarcopenia through carbonyl stress.Elife. 2023 Mar 23;12:e85289. doi: 10.7554/eLife.85289. Elife. 2023. PMID: 36951533 Free PMC article.
-
Redox Control of Proteolysis During Inactivity-Induced Skeletal Muscle Atrophy.Antioxid Redox Signal. 2020 Sep 10;33(8):559-569. doi: 10.1089/ars.2019.8000. Epub 2020 Feb 24. Antioxid Redox Signal. 2020. PMID: 31941357 Free PMC article. Review.
-
Oxidative stress and disuse muscle atrophy: cause or consequence?Curr Opin Clin Nutr Metab Care. 2012 May;15(3):240-5. doi: 10.1097/MCO.0b013e328352b4c2. Curr Opin Clin Nutr Metab Care. 2012. PMID: 22466926 Free PMC article. Review.
-
Mechanistic links between oxidative stress and disuse muscle atrophy.Antioxid Redox Signal. 2011 Nov 1;15(9):2519-28. doi: 10.1089/ars.2011.3973. Epub 2011 Jun 17. Antioxid Redox Signal. 2011. PMID: 21457104 Free PMC article. Review.
-
Mechanisms of disuse muscle atrophy: role of oxidative stress.Am J Physiol Regul Integr Comp Physiol. 2005 Feb;288(2):R337-44. doi: 10.1152/ajpregu.00469.2004. Am J Physiol Regul Integr Comp Physiol. 2005. PMID: 15637170 Review.
Cited by
-
Multi-tissue metabolomics reveal mtDNA- and diet-specific metabolite profiles in a mouse model of cardiometabolic disease.Redox Biol. 2025 Apr;81:103541. doi: 10.1016/j.redox.2025.103541. Epub 2025 Feb 14. Redox Biol. 2025. PMID: 39983345 Free PMC article.
-
Inhibition of the skeletal muscle Lands cycle ameliorates weakness induced by physical inactivity.J Cachexia Sarcopenia Muscle. 2024 Feb;15(1):319-330. doi: 10.1002/jcsm.13406. Epub 2023 Dec 20. J Cachexia Sarcopenia Muscle. 2024. PMID: 38123161 Free PMC article.
-
Lipid peroxidation and sarcopenia: molecular mechanisms and potential therapeutic approaches.Front Med (Lausanne). 2025 Feb 3;12:1525205. doi: 10.3389/fmed.2025.1525205. eCollection 2025. Front Med (Lausanne). 2025. PMID: 39963429 Free PMC article. Review.
-
Overexpression of manganese superoxide dismutase mitigates ACL injury-induced muscle atrophy, weakness and oxidative damage.Free Radic Biol Med. 2024 Feb 20;212:191-198. doi: 10.1016/j.freeradbiomed.2023.12.037. Epub 2023 Dec 26. Free Radic Biol Med. 2024. PMID: 38154571 Free PMC article.
-
Association between residual cholesterol and sarcopenia in American adults.Front Endocrinol (Lausanne). 2024 Nov 28;15:1461961. doi: 10.3389/fendo.2024.1461961. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39669500 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

