Generation of human colon organoids from healthy and inflammatory bowel disease mucosa
- PMID: 36301950
- PMCID: PMC9612551
- DOI: 10.1371/journal.pone.0276195
Generation of human colon organoids from healthy and inflammatory bowel disease mucosa
Abstract
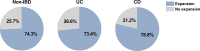
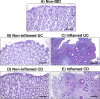
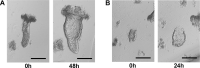
Ulcerative colitis and Crohn's disease are chronic inflammatory bowel diseases (IBD) of unknown cause characterized by a relapsing-remitting behavior. Growing evidence supports the idea that the epithelial barrier plays a central role in the pathogenesis of IBD as well as in its evolution over time, thus representing a potential target for novel therapeutic options. In the last decade, the introduction of 3D epithelial cultures from ex vivo-expanded intestinal adult stem cells (ASCs) has impacted our ability to study the function of the epithelium in several gastrointestinal disorders, including IBD. Here, we describe in detail a reproducible protocol to generate Matrigel-embedded epithelial organoids from ASCs of non-IBD and IBD donors using small colonic biopsies, including steps for its optimization. A slightly modified version of this protocol is also provided in case surgical samples are used. With this method, epithelial organoids can be expanded over several passages, thereby generating a large quantity of viable cells that can be used in multiple downstream analyses including genetic, transcriptional, proteomic and/or functional studies. In addition, 3D cultures generated using our protocol are suitable for the establishment of 2D cultures, which can model relevant cell-to-cell interactions that occur in IBD mucosa.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Organoid Medicine for Inflammatory Bowel Disease.Stem Cells. 2022 Mar 16;40(2):123-132. doi: 10.1093/stmcls/sxab020. Stem Cells. 2022. PMID: 35258629 Review.
-
Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases.World J Gastroenterol. 2019 Aug 14;25(30):4125-4147. doi: 10.3748/wjg.v25.i30.4125. World J Gastroenterol. 2019. PMID: 31435168 Free PMC article. Review.
-
Relevance of mouse and human IBD patient-derived colon organoids to investigate intestinal macrophage differentiation.J Leukoc Biol. 2025 Apr 23;117(4):qiaf004. doi: 10.1093/jleuko/qiaf004. J Leukoc Biol. 2025. PMID: 39832522
-
Modeling Inflammatory Bowel Disease by Intestinal Organoids.Recent Adv Inflamm Allergy Drug Discov. 2023;17(1):39-53. doi: 10.2174/2772270817666221121143853. Recent Adv Inflamm Allergy Drug Discov. 2023. PMID: 36411558 Review.
Cited by
-
Elevated risk of adverse effects from foodborne contaminants and drugs in inflammatory bowel disease: a review.Arch Toxicol. 2024 Nov;98(11):3519-3541. doi: 10.1007/s00204-024-03844-w. Epub 2024 Sep 9. Arch Toxicol. 2024. PMID: 39249550 Free PMC article. Review.
-
Characterization of patient-derived intestinal organoids for modelling fibrosis in Inflammatory Bowel Disease.Inflamm Res. 2024 Aug;73(8):1359-1370. doi: 10.1007/s00011-024-01901-9. Epub 2024 Jun 6. Inflamm Res. 2024. PMID: 38842554 Free PMC article.
-
Ion transport and epithelial barrier dysfunction in experimental models of ulcerative colitis.Am J Physiol Gastrointest Liver Physiol. 2025 Jun 1;328(6):G811-G830. doi: 10.1152/ajpgi.00204.2024. Epub 2025 Apr 4. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40184259 Free PMC article. Review.
-
A collection of patient-derived intestinal organoid lines reveals epithelial phenotypes associated with genetic drivers of pediatric inflammatory bowel disease.bioRxiv [Preprint]. 2025 Jun 16:2025.06.11.659052. doi: 10.1101/2025.06.11.659052. bioRxiv. 2025. PMID: 40666956 Free PMC article. Preprint.
-
Unraveling the impact of a germline heterozygous POLD1 frameshift variant in serrated polyposis syndrome.Front Mol Biosci. 2023 Jan 23;10:1119900. doi: 10.3389/fmolb.2023.1119900. eCollection 2023. Front Mol Biosci. 2023. PMID: 36756361 Free PMC article.

