Collagen cross-links scale with passive stiffness in dystrophic mouse muscles, but are not altered with administration of a lysyl oxidase inhibitor
- PMID: 36302059
- PMCID: PMC9612445
- DOI: 10.1371/journal.pone.0271776
Collagen cross-links scale with passive stiffness in dystrophic mouse muscles, but are not altered with administration of a lysyl oxidase inhibitor
Abstract
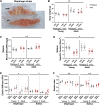
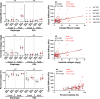
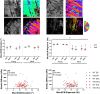
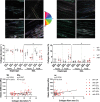
In Duchenne muscular dystrophy (DMD), a lack of functional dystrophin leads to myofiber instability and progressive muscle damage that results in fibrosis. While fibrosis is primarily characterized by an accumulation of extracellular matrix (ECM) components, there are changes in ECM architecture during fibrosis that relate more closely to functional muscle stiffness. One of these architectural changes in dystrophic muscle is collagen cross-linking, which has been shown to increase the passive muscle stiffness in models of fibrosis including the mdx mouse, a model of DMD. We tested whether the intraperitoneal injections of beta-aminopropionitrile (BAPN), an inhibitor of the cross-linking enzyme lysyl oxidase, would reduce collagen cross-linking and passive stiffness in young and adult mdx mice compared to saline-injected controls. We found no significant differences between BAPN treated and saline treated mice in collagen cross-linking and stiffness parameters. However, we observed that while collagen cross-linking and passive stiffness scaled positively in dystrophic muscles, collagen fiber alignment scaled with passive stiffness distinctly between muscles. We also observed that the dystrophic diaphragm showed the most dramatic fibrosis in terms of collagen content, cross-linking, and stiffness. Overall, we show that while BAPN was not effective at reducing collagen cross-linking, the positive association between collagen cross-linking and stiffness in dystrophic muscles still show cross-linking as a viable target for reducing passive muscle stiffness in DMD or other fibrotic muscle conditions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

