Severity of COVID-19 among solid organ transplant recipients in Canada, 2020-2021: a prospective, multicentre cohort study
- PMID: 36302101
- PMCID: PMC9435532
- DOI: 10.1503/cmaj.220620
Severity of COVID-19 among solid organ transplant recipients in Canada, 2020-2021: a prospective, multicentre cohort study
Abstract
Background: Severe COVID-19 appears to disproportionately affect people who are immunocompromised, although Canadian data in this context are limited. We sought to determine factors associated with severe COVID-19 outcomes among recipients of organ transplants across Canada.
Methods: We performed a multicentre, prospective cohort study of all recipients of solid organ transplants from 9 transplant programs in Canada who received a diagnosis of COVID-19 from March 2020 to November 2021. Data were analyzed to determine risk factors for oxygen requirement and other metrics of disease severity. We compared outcomes by organ transplant type and examined changes in outcomes over time. We performed a multivariable analysis to determine variables associated with need for supplemental oxygen.
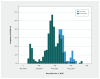
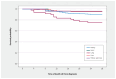
Results: A total of 509 patients with solid organ transplants had confirmed COVID-19 during the study period. Risk factors associated with needing (n = 190), compared with not needing (n = 319), supplemental oxygen included age (median 62.6 yr, interquartile range [IQR] 52.5-69.5 yr v. median 55.5 yr, IQR 47.5-66.5; p < 0.001) and number of comorbidities (median 3, IQR 2-3 v. median 2, IQR 1-3; p < 0.001), as well as parameters associated with immunosuppression. Recipients of lung transplants (n = 48) were more likely to have severe disease with a high mortality rate (n = 15, 31.3%) compared with recipients of other organ transplants, including kidney (n = 48, 14.8%), heart (n = 1, 4.4%), liver (n = 9, 11.4%) and kidney-pancreas (n = 3, 12.0%) transplants (p = 0.02). Protective factors against needing supplemental oxygen included having had a liver transplant and receiving azathioprine. Having had 2 doses of SARS-CoV-2 vaccine did not have an appreciable influence on oxygen requirement. Multivariable analysis showed that older age (odds ratio [OR] 1.04, 95% confidence interval [CI] 1.02-1.07) and number of comorbidities (OR 1.63, 95% CI 1.30-2.04), among other factors, were associated with the need for supplemental oxygen. Over time, disease severity did not decline significantly.
Interpretation: Despite therapeutic advances and vaccination of recipients of solid organ transplants, evidence of increased severity of COVID-19, in particular among those with lung transplants, supports ongoing public health measures to protect these at-risk people, and early use of COVID-19 therapies for recipients of solid organ transplants.
© 2022 CMA Impact Inc. or its licensors.
Conflict of interest statement
Competing interests: Geneviève Huard reports payment from Gilead and AbbVie, and participation on a data safety monitoring board with Paladin. Ruth Sapir-Pichhadze reports grants from the Canadian Institutes of Health Research, Fonds de recherche du Québec, Genome Canada and Kidney Foundation of Canada, as well as speaker fees from the Korean Society of Nephrology. Atul Humar reports fees from Roche, Astellas and Merck. Deepali Kumar reports grants from Roche, GSK, Takeda, Qiagen, Amplyx and Atara Biotherapeutics, as well as fees from Roche, GSK, Takeda, Exevir, Astellas and Meducom. Deepali Kumar is the 2022–2023 president of the American Society of Transplantation. No other competing interests were declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
