Increased HIV-1 infection in PBMCs treated in vitro with menstrual cycle phase hormones or medroxyprogesterone acetate likely occurs via different mechanisms
- PMID: 36302121
- PMCID: PMC9884997
- DOI: 10.1111/aji.13643
Increased HIV-1 infection in PBMCs treated in vitro with menstrual cycle phase hormones or medroxyprogesterone acetate likely occurs via different mechanisms
Abstract
Problem: Both luteal phase progesterone (P4) levels and use of the intramuscular (IM) injectable progestin-only contraceptive depo-medroxyprogesterone acetate (DMPA-IM) have been linked to increased S/HIV acquisition in animal, clinical and in vitro models. Several plausible mechanisms could explain MPA-induced HIV-1 acquisition while those for the luteal phase are underexplored.
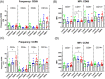
Method of study: Peripheral blood mononuclear cells (PBMCs) were treated with P4 and estrogen at concentrations mimicking the luteal phase, follicular phase or with levels of MPA mimicking peak serum levels in DMPA-IM users. Cells were infected with an R5-tropic infectious molecular clone and HIV-1 infection was measured. A role for the glucocorticoid receptor (GR) was investigated using the GR/PR antagonist RU486. CCR5 protein levels and activation status, assessed by levels of the activation marker CD69, were measured by flow cytometry after treatment in vitro and in PBMCs from naturally-cycling women or DMPA-IM users.
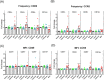
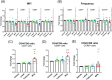
Results: Both MPA and luteal phase hormones significantly increased HIV-1 infection in vitro. However, MPA but not luteal phase hormones increased the CD4+/CD8+ T cell ratio, CCR5 protein expression on CD4+ T cells and increased expression of the activation marker CD69. The GR is involved in MPA-induced, but not luteal phase hormone-induced increased HIV-1 infection. In DMPA-IM users, the frequency of CCR5-expressing CD3+ and CD8+ cells was higher than for women in the luteal phase.
Conclusions: MPA increases HIV-1 infection in a manner different from that of luteal phase hormones, most likely involving the GR and at least in part changes in the frequency and/or expression of CCR5 and CD69.
Keywords: CCR5; HIV-1; PBMCs; glucocorticoid receptor; luteal phase; medroxyprogesterone acetate; menstrual cycle.
© 2022 The Authors. American Journal of Reproductive Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63(6):544‐565. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

