Randomized Phase II Trial of Sunitinib or Cediranib in Alveolar Soft Part Sarcoma
- PMID: 36302173
- PMCID: PMC10068440
- DOI: 10.1158/1078-0432.CCR-22-2145
Randomized Phase II Trial of Sunitinib or Cediranib in Alveolar Soft Part Sarcoma
Abstract
Purpose: Alveolar soft part sarcoma (ASPS) is a rare, highly vascular tumor with few treatment options. We designed a phase II randomized trial to determine the activity and tolerability of single-agent cediranib or sunitinib in patients with advanced metastatic ASPS.
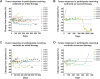
Patients and methods: Patients 16 years of age and older were randomized to receive cediranib (30 mg) or sunitinib (37.5 mg) in 28-day cycles. Patients could cross over to the other treatment arm at disease progression. The primary endpoint was to measure the objective response rate (ORR) for each agent. Median progression-free survival (mPFS) for the two arms was also determined.
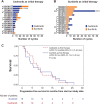
Results: Twenty-nine of 34 enrolled patients were evaluable for response. One patient on each of the initial two treatment arms had a partial response (ORR: 6.7% and 7.1% for cediranib and sunitinib, respectively). Twenty-four patients had a best response of stable disease (86.7% and 78.6% for cediranib and sunitinib, respectively). There were no significant differences in mPFS for the two treatment arms. Clinical benefit (i.e., objective response or stable disease for a minimum of four or six cycles of therapy) on the first-line tyrosine kinase inhibitor (TKI) therapy did not predict benefit on the second-line TKI. Both drugs were well tolerated. As of August 2021, 1 patient (unevaluable for ORR) remains on study.
Conclusions: The study did not meet its endpoints for ORR. Although both TKIs provided clinical benefit, the outcomes may have been attenuated in patients who had progressed ≤6 months before enrollment, potentially accounting for the low response rates. See related commentary by Wilky and Maleddu, p. 1163.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
-
Much Ado about ASPS: The Rapidly Changing Treatment Paradigms of 2022.Clin Cancer Res. 2023 Apr 3;29(7):1163-1166. doi: 10.1158/1078-0432.CCR-22-3399. Clin Cancer Res. 2023. PMID: 36692840
Comment on
-
Much Ado about ASPS: The Rapidly Changing Treatment Paradigms of 2022.Clin Cancer Res. 2023 Apr 3;29(7):1163-1166. doi: 10.1158/1078-0432.CCR-22-3399. Clin Cancer Res. 2023. PMID: 36692840
References
-
- Flores RJ, Harrison DJ, Federman NC, Furman WL, Huh WW, Broaddus EG, et al. . Alveolar soft part sarcoma in children and young adults: a report of 69 cases. Pediatr Blood Cancer 2018;65:e26953. - PubMed
-
- Paoluzzi L, Maki RG. Diagnosis, prognosis, and treatment of alveolar soft-part sarcoma: a review. JAMA Oncol 2019;5:254–60. - PubMed
-
- Portera CA Jr, Ho V, Patel SR, Hunt KK, Feig BW, Respondek PM, et al. . Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer 2001;91:585–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

