Senescence Rewires Microenvironment Sensing to Facilitate Antitumor Immunity
- PMID: 36302222
- PMCID: PMC9901536
- DOI: 10.1158/2159-8290.CD-22-0528
Senescence Rewires Microenvironment Sensing to Facilitate Antitumor Immunity
Abstract
Cellular senescence involves a stable cell-cycle arrest coupled to a secretory program that, in some instances, stimulates the immune clearance of senescent cells. Using an immune-competent liver cancer model in which senescence triggers CD8 T cell-mediated tumor rejection, we show that senescence also remodels the cell-surface proteome to alter how tumor cells sense environmental factors, as exemplified by type II interferon (IFNγ). Compared with proliferating cells, senescent cells upregulate the IFNγ receptor, become hypersensitized to microenvironmental IFNγ, and more robustly induce the antigen-presenting machinery-effects also recapitulated in human tumor cells undergoing therapy-induced senescence. Disruption of IFNγ sensing in senescent cells blunts their immune-mediated clearance without disabling the senescence state or its characteristic secretory program. Our results demonstrate that senescent cells have an enhanced ability to both send and receive environmental signals and imply that each process is required for their effective immune surveillance.
Significance: Our work uncovers an interplay between tissue remodeling and tissue-sensing programs that can be engaged by senescence in advanced cancers to render tumor cells more visible to the adaptive immune system. This new facet of senescence establishes reciprocal heterotypic signaling interactions that can be induced therapeutically to enhance antitumor immunity. See related article by Marin et al., p. 410. This article is highlighted in the In This Issue feature, p. 247.
©2022 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
R. Mezzadra reports grants from the Cancer Research Institute and NWO (Dutch Research Council) during the conduct of the study. N. Bernstein reports former employment with Calico Labs, which is where he worked at the time he contributed to this article, as well as current employment with TenSixteen Bio. M. Egeblad reports personal fees from Insmed and Vividion Therapeutics, and other support from Agios outside the submitted work. D. Alonso-Curbelo reports nonfinancial support from Calico Life Sciences during the conduct of the study. S.W. Lowe reports grants from Calico Life Sciences and nonfinancial support from Calico Life Sciences during the conduct of the study, as well as the following relationships not directly related to this work: consulting for and equity in Oric Pharmaceuticals, Blueprint Medicines, Mirimus Inc., Senecea Therapeutics, Faeth Therapeutics, and PMV Pharmaceuticals. No disclosures were reported by the other authors.
Figures
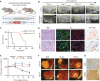
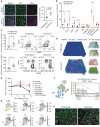
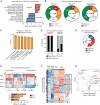

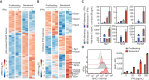


Comment in
-
Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity.Cancer Discov. 2023 Feb 6;13(2):410-431. doi: 10.1158/2159-8290.CD-22-0523. Cancer Discov. 2023. PMID: 36302218 Free PMC article.
-
When the tumour is at bay, the CD8+ T cells come out to play.Nat Rev Cancer. 2023 Jan;23(1):5. doi: 10.1038/s41568-022-00540-8. Nat Rev Cancer. 2023. PMID: 36456754 No abstract available.
Comment on
-
Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity.Cancer Discov. 2023 Feb 6;13(2):410-431. doi: 10.1158/2159-8290.CD-22-0523. Cancer Discov. 2023. PMID: 36302218 Free PMC article.
References
-
- Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014;15:482–96. - PubMed
-
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic Ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997;88:593–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

