Health Care Utilization and Costs in Systemic Therapies for Metastatic Melanoma from 2016 to 2020
- PMID: 36302223
- PMCID: PMC10020812
- DOI: 10.1093/oncolo/oyac219
Health Care Utilization and Costs in Systemic Therapies for Metastatic Melanoma from 2016 to 2020
Abstract
Background: Widespread implementation of immune checkpoint inhibitors (ICI) and targeted therapies for metastatic melanoma has led to a decline in melanoma-related mortality but increased healthcare costs. We aimed to determine how healthcare utilization varied by systemic, non-adjuvant melanoma treatment from 2016 to 2020.
Patients and methods: Adults with presumed stage IV metastatic melanoma receiving systemic therapy from 2016 to 2020 were identified in Optum, a nationwide commercial claims database. Treatment groups were nivolumab, pembrolizumab, ipilimumab+nivolumab (combination-ICI), or BRAF+MEK inhibitor (BRAFi+MEKi) therapy. Outcomes included hospitalizations, days hospitalized, emergency room (ER) visits, outpatient visits, and healthcare costs per patient per month (pppm). Multivariable regression models were used to analyze whether cost and utilization outcomes varied by treatment group, with nivolumab as reference.
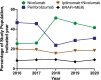
Results: Among 2018 adult patients with metastatic melanoma identified, mean (SD) age was 67 (15) years. From 2016 to 2020, nivolumab surpassed pembrolizumab as the most prescribed systemic melanoma therapy while combination-ICI and BRAFi+MEKi therapies remained stable. Relative to nivolumab, all other therapies were associated with increased total healthcare costs (combination-ICI: β = $47 600 pppm, 95%CI $42 200-$53 100; BRAFi+MEKi: β = $3810, 95%CI $365-$7260; pembrolizumab: β = $6450, 95%CI $4420-$8480). Combination-ICI and BRAFi+MEKi therapies were associated with more inpatient hospital days.
Conclusions: Amid the evolving landscape of systemic therapy for advanced melanoma, nivolumab monotherapy emerged as the most used and least costly systemic treatment from 2016 to 2020. Its sharp increase in use in 2018 and lower costs relative to pembrolizumab may in part be due to earlier adoption of less frequent dosing intervals.
Keywords: administrative claims; healthcare; healthcare cost; immune checkpoint inhibitors; melanoma; molecular targeted therapy.
© The Author(s) 2022. Published by Oxford University Press.
Conflict of interest statement
Figures
Comment in
-
Trends, Changes, and Disruptions: The Fragile Economics of Cancer Treatments.Oncologist. 2023 Mar 17;28(3):193-195. doi: 10.1093/oncolo/oyac258. Oncologist. 2023. PMID: 36640143 Free PMC article.
Similar articles
-
Health Care Utilization and Costs Associated With Systemic First-Line Metastatic Melanoma Therapies in the United States.JCO Oncol Pract. 2022 Jan;18(1):e163-e174. doi: 10.1200/OP.21.00140. Epub 2021 Jul 6. JCO Oncol Pract. 2022. PMID: 34228489
-
Adverse effects of systemic advanced melanoma therapies-do BRAF/MEK inhibitors increase the incidence of mesenteric panniculitis?Eur Radiol. 2025 May 1. doi: 10.1007/s00330-025-11642-w. Online ahead of print. Eur Radiol. 2025. PMID: 40310541
-
Treatment-Free Survival After Nivolumab vs Pembrolizumab vs Nivolumab-Ipilimumab for Advanced Melanoma.JAMA Netw Open. 2023 Jun 1;6(6):e2319607. doi: 10.1001/jamanetworkopen.2023.19607. JAMA Netw Open. 2023. PMID: 37351883 Free PMC article.
-
Recent advances in therapeutic strategies for unresectable or metastatic melanoma and real-world data in Japan.Int J Clin Oncol. 2019 Dec;24(12):1508-1514. doi: 10.1007/s10147-018-1246-y. Epub 2018 Feb 22. Int J Clin Oncol. 2019. PMID: 29470725 Review.
-
Novel adjuvant options for cutaneous melanoma.Ann Oncol. 2021 Jul;32(7):854-865. doi: 10.1016/j.annonc.2021.03.198. Epub 2021 Mar 24. Ann Oncol. 2021. PMID: 33771664 Review.
Cited by
-
Trends, Changes, and Disruptions: The Fragile Economics of Cancer Treatments.Oncologist. 2023 Mar 17;28(3):193-195. doi: 10.1093/oncolo/oyac258. Oncologist. 2023. PMID: 36640143 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials