miR-214-5p/C1QTNF1 axis enhances PCV2 replication through promoting autophagy by targeting AKT/mTOR signaling pathway
- PMID: 36302471
- PMCID: PMC10194317
- DOI: 10.1016/j.virusres.2022.198990
miR-214-5p/C1QTNF1 axis enhances PCV2 replication through promoting autophagy by targeting AKT/mTOR signaling pathway
Abstract
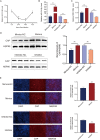
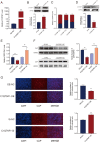
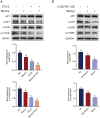
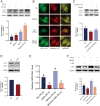
Porcine circovirus type 2 (PCV2) is the causative agent of PCV2-associated disease, which causes a relevant economic impact on the global swine industry. Accumulating data have indicated host microRNAs play essential roles in numerous virus replication of pigs, while their roles in PCV2 replication remain unclear. Herein, we demonstrated that PCV2 infection downregulated the expression of miR-214-5p in PK15 cells, and miR-214-5p promoted PCV2 replication. C1q/tumor necrosis factor-related protein 1 (C1QTNF1) was then identified as a target gene of miR-214-5p, and C1QTNF1 suppressed PCV2 replication. Interestingly, miR-214-5p/C1QTNF1 axis negatively regulated AKT/mTOR signaling, and then enhanced PCV2 replication through promoting autophagy in PK15 cells. Collectively, our findings provide insight into the mechanism of PCV2 replication and highlight miR-214-5p and C1QTNF1 as potential novel targets for the treatment of PCV2 infection.
Keywords: AKT/mTOR signaling pathway; Autophagy; C1QTNF1; PCV2; Pigs; miR-214–5p.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

