Key Residue for Aggregation of Amyloid-β Peptides
- PMID: 36302506
- PMCID: PMC9673141
- DOI: 10.1021/acschemneuro.2c00358
Key Residue for Aggregation of Amyloid-β Peptides
Abstract
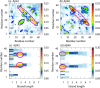
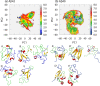
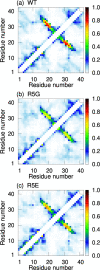
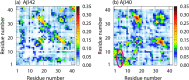
It is known that oligomers of amyloid-β (Aβ) peptide are associated with Alzheimer's disease. Aβ has two isoforms: Aβ40 and Aβ42. Although the difference between Aβ40 and Aβ42 is only two additional C-terminal residues, Aβ42 aggregates much faster than Aβ40. It is unknown what role the C-terminal two residues play in accelerating aggregation. Since Aβ42 is more toxic than Aβ40, its oligomerization process needs to be clarified. Moreover, clarifying the differences between the oligomerization processes of Aβ40 and Aβ42 is essential to elucidate the key factors of oligomerization. Therefore, to investigate the dimerization process, which is the early oligomerization process, Hamiltonian replica-permutation molecular dynamics simulations were performed for Aβ40 and Aβ42. We identified a key residue, Arg5, for the Aβ42 dimerization. The two additional residues in Aβ42 allow the C-terminus to form contact with Arg5 because of the electrostatic attraction between them, and this contact stabilizes the β-hairpin. This β-hairpin promotes dimer formation through the intermolecular β-bridges. Thus, we examined the effects of amino acid substitutions of Arg5, thereby confirming that the mutations remarkably suppressed the aggregation of Aβ42. Moreover, the mutations of Arg5 suppressed the Aβ40 aggregation. It was found by analyzing the simulations that Arg5 is important for Aβ40 to form intermolecular contacts. Thus, it was clarified that the role of Arg5 in the oligomerization process varies due to the two additional C-terminal residues.
Keywords: amyloid-β peptide; generalized-ensemble algorithm; molecular dynamics simulation; protein aggregation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Dissecting the Molecular Mechanisms of the Co-Aggregation of Aβ40 and Aβ42 Peptides: A REMD Simulation Study.J Phys Chem B. 2023 May 11;127(18):4050-4060. doi: 10.1021/acs.jpcb.3c01078. Epub 2023 May 1. J Phys Chem B. 2023. PMID: 37126408
-
In silico investigation on the inhibition of Aβ42 aggregation by Aβ40 peptide by potential of mean force study.J Biomol Struct Dyn. 2018 Feb;36(3):741-752. doi: 10.1080/07391102.2017.1296783. Epub 2017 Mar 13. J Biomol Struct Dyn. 2018. PMID: 28278027
-
Effects of the Arctic (E22-->G) mutation on amyloid beta-protein folding: discrete molecular dynamics study.J Am Chem Soc. 2008 Dec 24;130(51):17413-22. doi: 10.1021/ja804984h. J Am Chem Soc. 2008. PMID: 19053400
-
Distinctive contribution of two additional residues in protein aggregation of Aβ42 and Aβ40 isoforms.BMB Rep. 2024 Jun;57(6):263-272. doi: 10.5483/BMBRep.2024-0044. BMB Rep. 2024. PMID: 38835114 Free PMC article. Review.
-
Aβ42 and Aβ40: similarities and differences.J Pept Sci. 2015 Jul;21(7):522-9. doi: 10.1002/psc.2789. Epub 2015 May 28. J Pept Sci. 2015. PMID: 26018760 Review.
Cited by
-
Decoding Solubility Signatures from Amyloid Monomer Energy Landscapes.J Chem Theory Comput. 2025 Mar 11;21(5):2736-2756. doi: 10.1021/acs.jctc.4c01623. Epub 2025 Feb 24. J Chem Theory Comput. 2025. PMID: 39988900 Free PMC article.
-
Sugar distributions on gangliosides guide the formation and stability of amyloid-β oligomers.Biophys Chem. 2023 Sep;300:107073. doi: 10.1016/j.bpc.2023.107073. Epub 2023 Jun 30. Biophys Chem. 2023. PMID: 37413816 Free PMC article.
-
Helix-to-sheet transition of the Aβ42 peptide revealed using an enhanced sampling strategy and Markov state model.Comput Struct Biotechnol J. 2023 Dec 19;23:688-699. doi: 10.1016/j.csbj.2023.12.015. eCollection 2024 Dec. Comput Struct Biotechnol J. 2023. PMID: 38292476 Free PMC article.
-
Inhibition Effect and Molecular Mechanisms of Quercetin on the Aβ42 Dimer: A Molecular Dynamics Simulation Study.ACS Omega. 2023 May 9;8(20):18009-18018. doi: 10.1021/acsomega.3c01208. eCollection 2023 May 23. ACS Omega. 2023. PMID: 37251196 Free PMC article.
-
A β-hairpin peptide derived from Aβ forms different oligomers in the crystal state and in aqueous solution.Org Biomol Chem. 2025 Apr 16;23(16):3881-3893. doi: 10.1039/d5ob00296f. Org Biomol Chem. 2025. PMID: 40130612
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

