Engagement and attrition with eHealth tools for remote monitoring in chronic arthritis: a systematic review and meta-analysis
- PMID: 36302561
- PMCID: PMC9621170
- DOI: 10.1136/rmdopen-2022-002625
Engagement and attrition with eHealth tools for remote monitoring in chronic arthritis: a systematic review and meta-analysis
Abstract
Objectives: Although eHealth tools are potentially useful for remote disease monitoring, barriers include concerns of low engagement and high attrition. We aimed to summarise evidence on patients' engagement and attrition with eHealth tools for remotely monitoring disease activity/impact in chronic arthritis.
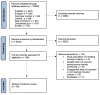
Methods: A systematic literature search was conducted for original articles and abstracts published before September 2022. Eligible studies reported quantitative measures of patients' engagement with eHealth instruments used for remote monitoring in chronic arthritis. Engagement rates were pooled using random effects meta-analysis.
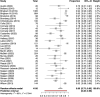
Results: Of 8246 references, 45 studies were included: 23 using smartphone applications, 13 evaluating wearable activity trackers, 7 using personal digital assistants, 6 including web-based platforms and 2 using short message service. Wearable-based studies mostly reported engagement as the proportion of days the tracker was worn (70% pooled across 6 studies). For other eHealth tools, engagement was mostly reported as completion rates for remote patient-reported outcomes (PROs). The pooled completion rate was 80%, although between-study heterogeneity was high (I2 93%) with significant differences between eHealth tools and frequency of PRO-collection. Engagement significantly decreased with longer study duration, but attrition varied across studies (0%-89%). Several predictors of higher engagement were reported. Data on the influence of PRO-reporting frequency were conflicting.
Conclusion: Generally high patient engagement was reported with eHealth tools for remote monitoring in chronic arthritis. However, we found considerable between-study heterogeneity and a relative lack of real-world data. Future studies should use standardised measures of engagement, preferably assessed in a daily practice setting.
Trial registeration number: The protocol was registered on PROSPERO (CRD42021267936).
Keywords: arthritis; epidemiology; health services research; patient reported outcome measures.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Similar articles
-
The Use and Effects of Electronic Health Tools for Patient Self-Monitoring and Reporting of Outcomes Following Medication Use: Systematic Review.J Med Internet Res. 2018 Dec 18;20(12):e294. doi: 10.2196/jmir.9284. J Med Internet Res. 2018. PMID: 30563822 Free PMC article.
-
Impact of summer programmes on the outcomes of disadvantaged or 'at risk' young people: A systematic review.Campbell Syst Rev. 2024 Jun 13;20(2):e1406. doi: 10.1002/cl2.1406. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38873396 Free PMC article. Review.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Examining Predictors of Real-World User Engagement with Self-Guided eHealth Interventions: Analysis of Mobile Apps and Websites Using a Novel Dataset.J Med Internet Res. 2018 Dec 14;20(12):e11491. doi: 10.2196/11491. J Med Internet Res. 2018. PMID: 30552077 Free PMC article.
-
Utilizing Digital Health to Collect Electronic Patient-Reported Outcomes in Prostate Cancer: Single-Arm Pilot Trial.J Med Internet Res. 2020 Mar 25;22(3):e12689. doi: 10.2196/12689. J Med Internet Res. 2020. PMID: 32209536 Free PMC article.
Cited by
-
Effectiveness and feasibility of a mobile health self-management intervention in rheumatoid arthritis: study protocol for a pragmatic multicentre randomised controlled trial (AEGORA).Trials. 2023 Oct 28;24(1):697. doi: 10.1186/s13063-023-07733-y. Trials. 2023. PMID: 37898781 Free PMC article.
-
Reporting quality of published reviews of commercial and publicly available mobile health apps (mHealth app reviews): a scoping review protocol.BMJ Open. 2024 Jul 4;14(7):e083364. doi: 10.1136/bmjopen-2023-083364. BMJ Open. 2024. PMID: 38964792 Free PMC article.
-
Efficacy of a cognitive-behavioral digital therapeutic on psychosocial outcomes in rheumatoid arthritis: randomized controlled trial.Npj Ment Health Res. 2024 Sep 3;3(1):41. doi: 10.1038/s44184-024-00085-8. Npj Ment Health Res. 2024. PMID: 39227501 Free PMC article.
-
Mobile and Web-Based Partnered Intervention to Improve Remote Access to Pain and Posttraumatic Stress Disorder Symptom Management: Recruitment and Attrition in a Randomized Controlled Trial.J Med Internet Res. 2023 Oct 3;25:e49678. doi: 10.2196/49678. J Med Internet Res. 2023. PMID: 37788078 Free PMC article. Clinical Trial.
-
Implementation-effectiveness of the power over pain portal for patients awaiting a tertiary care consultation for chronic pain: A pilot feasibility study.Digit Health. 2025 Mar 17;11:20552076251326229. doi: 10.1177/20552076251326229. eCollection 2025 Jan-Dec. Digit Health. 2025. PMID: 40103642 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
