Genomic Disorders in CKD across the Lifespan
- PMID: 36302597
- PMCID: PMC10103259
- DOI: 10.1681/ASN.2022060725
Genomic Disorders in CKD across the Lifespan
Abstract
Significance statement: Pathogenic structural genetic variants, also known as genomic disorders, have been associated with pediatric CKD. This study extends those results across the lifespan, with genomic disorders enriched in both pediatric and adult patients compared with controls. In the Chronic Renal Insufficiency Cohort study, genomic disorders were also associated with lower serum Mg, lower educational performance, and a higher risk of death. A phenome-wide association study confirmed the link between kidney disease and genomic disorders in an unbiased way. Systematic detection of genomic disorders can provide a molecular diagnosis and refine prediction of risk and prognosis.
Background: Genomic disorders (GDs) are associated with many comorbid outcomes, including CKD. Identification of GDs has diagnostic utility.
Methods: We examined the prevalence of GDs among participants in the Chronic Kidney Disease in Children (CKiD) cohort II ( n =248), Chronic Renal Insufficiency Cohort (CRIC) study ( n =3375), Columbia University CKD Biobank (CU-CKD; n =1986), and the Family Investigation of Nephropathy and Diabetes (FIND; n =1318) compared with 30,746 controls. We also performed a phenome-wide association analysis (PheWAS) of GDs in the electronic MEdical Records and GEnomics (eMERGE; n =11,146) cohort.
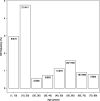
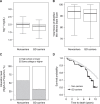
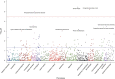
Results: We found nine out of 248 (3.6%) CKiD II participants carried a GD, replicating prior findings in pediatric CKD. We also identified GDs in 72 out of 6679 (1.1%) adult patients with CKD in the CRIC, CU-CKD, and FIND cohorts, compared with 199 out of 30,746 (0.65%) GDs in controls (OR, 1.7; 95% CI, 1.3 to 2.2). Among adults with CKD, we found recurrent GDs at the 1q21.1, 16p11.2, 17q12, and 22q11.2 loci. The 17q12 GD (diagnostic of renal cyst and diabetes syndrome) was most frequent, present in 1:252 patients with CKD and diabetes. In the PheWAS, dialysis and neuropsychiatric phenotypes were the top associations with GDs. In CRIC participants, GDs were associated with lower serum magnesium, lower educational achievement, and higher mortality risk.
Conclusion: Undiagnosed GDs are detected both in children and adults with CKD. Identification of GDs in these patients can enable a precise genetic diagnosis, inform prognosis, and help stratify risk in clinical studies. GDs could also provide a molecular explanation for nephropathy and comorbidities, such as poorer neurocognition for a subset of patients.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
A.G. Gharavi reports having consultancy agreements with Actio Biosciences, AstraZeneca Center for Genomics Research, Goldfinch Bio, Natera, Novartis, and Travere; reports having an ownership interest in Actio Biosciences; reports receiving research funding from Natera and the Renal Research Institute; reports receiving honoraria from Alnylam and Sanofi; and reports having an advisory or leadership role with the editorial boards of
Figures

Comment in
-
Kidney Genetics: Continuing Discoveries and a Roadmap to the Clinic.J Am Soc Nephrol. 2023 Apr 1;34(4):519-520. doi: 10.1681/ASN.0000000000000077. Epub 2023 Feb 9. J Am Soc Nephrol. 2023. PMID: 36758119 Free PMC article.
References
-
- Fox CS, Yang Q, Cupples LA, Guo CY, Larson MG, Leip EP, et al. : Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: The Framingham Heart Study. J Am Soc Nephrol 15: 2457–2461, 2004 - PubMed
-
- Connaughton DM, Bukhari S, Conlon P, Cassidy E, O’Toole M, Mohamad M, et al. : The Irish Kidney Gene Project--Prevalence of family history in patients with kidney disease in Ireland. Nephron 130: 293–301, 2015 - PubMed
-
- McClellan WM, Satko SG, Gladstone E, Krisher JO, Narva AS, Freedman BI: Individuals with a family history of ESRD are a high-risk population for CKD: Implications for targeted surveillance and intervention activities. Am J Kidney Dis 53[Suppl 3]: S100–S106, 2009 - PubMed
-
- Devuyst O Knoers NV Remuzzi G Schaefer F; Board of the Working Group for Inherited Kidney Diseases of the European Renal Association and European Dialysis and Transplant Association : Rare inherited kidney diseases: Challenges, opportunities, and perspectives. Lancet 383: 1844–1859, 2014 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

