Early Effect of the Circular Model of Kidney Allocation in the United States
- PMID: 36302599
- PMCID: PMC10101588
- DOI: 10.1681/ASN.2022040471
Early Effect of the Circular Model of Kidney Allocation in the United States
Abstract
Background: In March 2021, the United States implemented a new kidney allocation system (KAS250) for deceased donor kidney transplantation (DDKT), which eliminated the donation service area-based allocation and replaced it with a system on the basis of distance from donor hospital to transplant center within/outside a radius of 250 nautical miles. The effect of this policy on kidney discards and logistics is unknown.
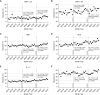
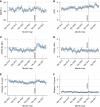
Methods: We examined discards, donor-recipient characteristics, cold ischemia time (CIT), and delayed graft function (DGF) during the first 9 months of KAS250 compared with a pre-KAS250 cohort from the preceding 2 years. Changes in discards and CIT after the onset of COVID-19 and the implementation of KAS250 were evaluated using an interrupted time-series model. Changes in allocation practices (biopsy, machine perfusion, and virtual cross-match) were also evaluated.
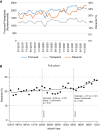
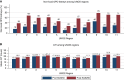
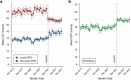
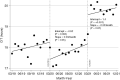
Results: Post-KAS250 saw a two-fold increase in kidneys imported from nonlocal organ procurement organizations (OPO) and a higher proportion of recipients with calculated panel reactive antibody (cPRA) 81%-98% (12% versus 8%; P <0.001) and those with >5 years of pretransplant dialysis (35% versus 33%; P <0.001). CIT increased (mean 2 hours), including among local OPO kidneys. DGF was similar on adjusted analysis. Discards after KAS250 did not immediately change, but we observed a statistically significant increase over time that was independent of donor quality. Machine perfusion use decreased, whereas reliance on virtual cross-match increased, which was associated with shorter CIT.
Conclusions: Early trends after KAS250 show an increase in transplant access to patients with cPRA>80% and those with longer dialysis duration, but this was accompanied by an increase in CIT and a suggestion of worsening kidney discards.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
V. Gunabushanam reports that his spouse is employed by Fitch Ratings. S. Hariharan reports being the owner of Transplant Interface, LLC. C. Puttarajappa reports research funding from NIH (NIDDK) and that his spouse is a site Primary Investigator for contracted clinical research with Abeona/Ultragenyx, Denali, Regenxbio, and Shire. S. Mohan reports consultancy for Angion Biomedica, eGenesis, and HSAG; an advisory or leadership role for ASN Quality Committee (member), ETCLC (National Faculty Chair), Kidney International Reports (ISN; deputy editor), SRTR Review Committee (member), and UNOS data advisory committee (vice chair); and research funding from NIH (NIDDK, NIHMD and NIBIB) and the Kidney Transplant Collaborative. All remaining authors have nothing to disclose.
Figures


References
-
- Gustafson SWT, Wey A, Salkowski N, Pyke J, Thompson B, Audette K, et al. : KPSAM simulation data on effect of removing DSA and region from kidney/pancreas/kidney-pancreas organ allocation policy, 2019. Available at: https://optn.transplant.hrsa.gov/media/2985/ki2019_01_analysisreport.pdf. Accessed August 1, 2021
-
- Adler JT, Husain SA, King KL, Mohan S: Greater complexity and monitoring of the new kidney allocation system: Implications and unintended consequences of concentric circle kidney allocation on network complexity. Am J Transplant 21: 2007–2013, 2021 - PubMed
-
- Organ Procurement and Transplantation Network : Continuous Distribution: Creating a More Fair and Patient-focused System for Organ Allocation. Available at: https://optn.transplant.hrsa.gov/policies-bylaws/a-closer-look/continuou.... Accessed March 21, 2022
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

