Endothelial alpha globin is a nitrite reductase
- PMID: 36302779
- PMCID: PMC9613979
- DOI: 10.1038/s41467-022-34154-3
Endothelial alpha globin is a nitrite reductase
Abstract
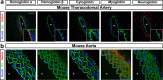
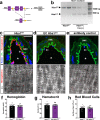
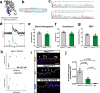
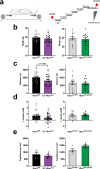
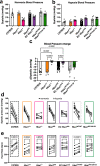
Resistance artery vasodilation in response to hypoxia is essential for matching tissue oxygen and demand. In hypoxia, erythrocytic hemoglobin tetramers produce nitric oxide through nitrite reduction. We hypothesized that the alpha subunit of hemoglobin expressed in endothelium also facilitates nitrite reduction proximal to smooth muscle. Here, we create two mouse strains to test this: an endothelial-specific alpha globin knockout (EC Hba1Δ/Δ) and another with an alpha globin allele mutated to prevent alpha globin's inhibitory interaction with endothelial nitric oxide synthase (Hba1WT/Δ36-39). The EC Hba1Δ/Δ mice had significantly decreased exercise capacity and intracellular nitrite consumption in hypoxic conditions, an effect absent in Hba1WT/Δ36-39 mice. Hypoxia-induced vasodilation is significantly decreased in arteries from EC Hba1Δ/Δ, but not Hba1WT/Δ36-39 mice. Hypoxia also does not lower blood pressure in EC Hba1Δ/Δ mice. We conclude the presence of alpha globin in resistance artery endothelium acts as a nitrite reductase providing local nitric oxide in response to hypoxia.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

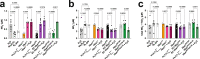

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

