Sarcomere dynamics revealed by a myofilament integrated FRET-based biosensor in live skeletal muscle fibers
- PMID: 36302792
- PMCID: PMC9613882
- DOI: 10.1038/s41598-022-21425-8
Sarcomere dynamics revealed by a myofilament integrated FRET-based biosensor in live skeletal muscle fibers
Abstract
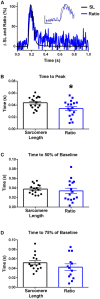
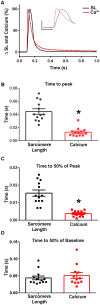
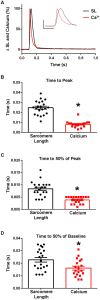
The sarcomere is the functional unit of skeletal muscle, essential for proper contraction. Numerous acquired and inherited myopathies impact sarcomere function causing clinically significant disease. Mechanistic investigations of sarcomere activation have been challenging to undertake in the context of intact, live skeletal muscle fibers during real time physiological twitch contractions. Here, a skeletal muscle specific, intramolecular FRET-based biosensor was designed and engineered into fast skeletal muscle troponin C (TnC) to investigate the dynamics of sarcomere activation. In transgenic animals, the TnC biosensor incorporated into the skeletal muscle fiber sarcomeres by stoichiometric replacement of endogenous TnC and did not alter normal skeletal muscle contractile form or function. In intact single adult skeletal muscle fibers, real time twitch contractile data showed the TnC biosensor transient preceding the peak amplitude of contraction. Importantly, under physiological temperatures, inactivation of the TnC biosensor transient decayed significantly more slowly than the Ca2+ transient and contraction. The uncoupling of the TnC biosensor transient from the Ca2+ transient indicates the biosensor is not functioning as a Ca2+ transient reporter, but rather reports dynamic sarcomere activation/ inactivation that, in turn, is due to the ensemble effects of multiple activating ligands within the myofilaments. Together, these findings provide the foundation for implementing this new biosensor in future physiological studies investigating the mechanism of activation of the skeletal muscle sarcomere in health and disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. - PubMed
-
- Gautel M, Djinović-Carugo K. The sarcomeric cytoskeleton: From molecules to motion. J. Exp. Biol. 2016;219:135–145. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

