Quiescent innate and adaptive immune responses maintain the long-term integrity of corneal endothelium reconstituted through allogeneic cell injection therapy
- PMID: 36302875
- PMCID: PMC9613641
- DOI: 10.1038/s41598-022-22522-4
Quiescent innate and adaptive immune responses maintain the long-term integrity of corneal endothelium reconstituted through allogeneic cell injection therapy
Abstract
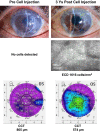
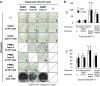
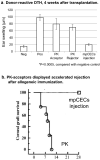
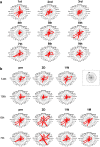
This study aims to clarify the immunogenicity in acquired and innate immune responses of cultured human corneal endothelial cells (hCECs) applied for cell injection therapy, a newly established modality for corneal endothelium failures. Thirty-four patients with corneal endothelial failure received injection of allogeneic hCEC suspension into anterior chamber. No sign of immunological rejection was observed in all 34 patients during the 5-8 years postoperative follow-up period. Cell injection therapy was successful in 2 patients treated for endothelial failure after penetrating keratoplasty and one patient with Descemet membrane stripping automated endothelial keratoplasty failure. ELISPOT assays performed in allo-mixed lymphocyte reaction to the alloantigen identical to that on the injected hCECs, elicited sparse IFN-γ-specific spots in the peripheral blood mononuclear cells of patients who received hCEC injection. The therapy generated simple and smooth graft-host junctions without wound stress. The injection of C57BL/6 CECs into the anterior chamber of BALB/c mice, which rejected C57BL/6 corneas 6 weeks ago, induced no sign of inflammatory reactions after the second challenge of alloantigen. Collectively, injection of the hCEC cell suspension in the aqueous humor induces immune tolerance that contributes to the survival of the reconstituted endothelium.
© 2022. The Author(s).
Conflict of interest statement
Dr. Kinoshita has received grants and personal fees from Santen Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Senju Pharmaceutical Co., Ltd. CorneaGen and Kowa Co., Ltd. outside the submitted work. He has received grants from HOYA Corporation, Oncolys Biopharma Inc., Alcon Japan, Ltd. and Abbott Medical Optics, Inc. outside the submitted work. He has received personal fees from Novartis, Aurion Biotech outside the submitted work. In addition, He has a patent licensed and a patent pending. Dr. Sotozono has received grants and personal fees from Santen Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd. and Senju Pharmaceutical Co., Ltd. outside the submitted work. She has been received grants from Alcon Japan, Ltd., Nitto Medic Co., Ltd., SEED Co., Ltd., Nitten Pharmaceutical Co., Ltd., CorneaGen, Hirosaki LI and Kowa Company outside the submitted work. She has received personal fees from Toa Pharmaceutical Co.,Ltd. outside the submitted work. Dr. Ueno has received a grant from Alcon Japan, Ltd. He has received personal fees from CorneaGen, Aurion Biotech and Tomey Corporation outside the submitted work; In addition, He has a patent licensed and a patent pending. The other authors declare no potential conflict of interest.
Figures
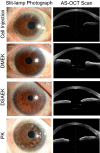
References
-
- Deng SX, et al. Descemet membrane endothelial keratoplasty: Safety and outcomes: A report by the American Academy of Ophthalmology. Ophthalmology. 2018;125:295–310. - PubMed
-
- Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. - PubMed
-
- Eye Bank Association of America. 2015 Eye Banking Statistical Report. (EBAA, Washington DC, 2016).
-
- Gain P, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167–173. - PubMed
-
- Melles GR, Ong TS, Ververs B, van der Wees J. Preliminary clinical results of Descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2008;145:222–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

