Adeno-associated virus-based caveolin-1 delivery via different routes for the prevention of cholesterol gallstone formation
- PMID: 36303150
- PMCID: PMC9609467
- DOI: 10.1186/s12944-022-01718-7
Adeno-associated virus-based caveolin-1 delivery via different routes for the prevention of cholesterol gallstone formation
Abstract
Background: Hepatic caveolin-1 (CAV1) is reduced in cholesterol gallstone disease (CGD). Mice with CAV1 deficiency were prone to develop CGD. However, it remains unknown whether restored hepatic CAV1 expression prevents the development of CGD.
Methods: C57BL/6 mice were injected with adeno-associated virus 2/8 (AAV2/8) vectors carrying the CAV1 gene (AAV2/8CAV1) via intravenous (i.v.) or intraperitoneal (i.p.) route and then subjected to a lithogenic diet (LD) for 8 weeks. Uninjected mice were used as controls. The functional consequences of rescuing CAV1 expression by either i.v. or i.p. AAV2/8CAV1 treatment for CGD prevention and its subsequent molecular mechanisms were examined.
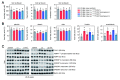
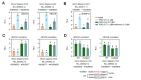
Results: CAV1 expression was reduced in the liver and gallbladder of LD-fed CGD mice. We discovered that AAV2/8CAV1 i.p. delivery results in higher transduction efficiency in the gallbladder than tail vein administration. Although either i.v. or i.p. injection of AAV2/8CAV1 improved liver lipid metabolic abnormalities in CGD mice but did not affect LD feeding-induced bile cholesterol supersaturation. In comparison with i.v. administration route, i.p. administration of AAV2/8CAV1 obviously increased CAV1 protein levels in the gallbladder of LD-fed mice, and i.p. delivery of AAV2/8CAV1 partially improved gallbladder cholecystokinin receptor (CCKAR) responsiveness and impeded bile cholesterol nucleation via the activation of adenosine monophosphate-activated protein kinase (AMPK) signaling, which induced a reduction in gallbladder mucin-1 (MUC1) and MUC5ac expression and gallbladder cholesterol accumulation.
Conclusion: CGD prevention by i.p. AAV2/8CAV1 injection in LD-fed mice was associated with the improvement of gallbladder stasis, which again supported the notion that supersaturated bile is required but not sufficient for the formation of cholesterol gallstones. Additionally, AAV treatment via the local i.p. injection offers particular advantages over the systemic i.v. route for much more effective gallbladder gene delivery, which will be an excellent tool for conducting preclinical functional studies on the maintenance of normal gallbladder function to prevent CGD.
Keywords: Adenosine monophosphate-activated protein kinase; Cholesterol gallstone disease; caveolin-1; mucin-1; mucin-5ac.
© 2022. The Author(s).
Conflict of interest statement
The authors do not have any disclosures to report.
Figures



Similar articles
-
A Gallbladder-Specific Hydrophobic Bile Acid-FXR-MUC1 Signaling Axis Mediates Cholesterol Gallstone Formation.Adv Sci (Weinh). 2025 Apr;12(13):e2401956. doi: 10.1002/advs.202401956. Epub 2025 Feb 11. Adv Sci (Weinh). 2025. PMID: 39932450 Free PMC article.
-
CAV1 Prevents Gallbladder Cholesterol Crystallization by Regulating Biosynthesis and Transport of Bile Salts.J Cell Biochem. 2016 Sep;117(9):2118-27. doi: 10.1002/jcb.25518. Epub 2016 Feb 29. J Cell Biochem. 2016. PMID: 26875794
-
Association of caveolin-3 and cholecystokinin A receptor with cholesterol gallstone disease in mice.World J Gastroenterol. 2014 Jul 28;20(28):9513-8. doi: 10.3748/wjg.v20.i28.9513. World J Gastroenterol. 2014. PMID: 25071346 Free PMC article.
-
An Update on the Lithogenic Mechanisms of Cholecystokinin a Receptor (CCKAR), an Important Gallstone Gene for Lith13.Genes (Basel). 2020 Nov 29;11(12):1438. doi: 10.3390/genes11121438. Genes (Basel). 2020. PMID: 33260332 Free PMC article. Review.
-
Triglycerides and gallstone formation.Clin Chim Acta. 2010 Nov 11;411(21-22):1625-31. doi: 10.1016/j.cca.2010.08.003. Epub 2010 Aug 10. Clin Chim Acta. 2010. PMID: 20699090 Review.
Cited by
-
A Gallbladder-Specific Hydrophobic Bile Acid-FXR-MUC1 Signaling Axis Mediates Cholesterol Gallstone Formation.Adv Sci (Weinh). 2025 Apr;12(13):e2401956. doi: 10.1002/advs.202401956. Epub 2025 Feb 11. Adv Sci (Weinh). 2025. PMID: 39932450 Free PMC article.
-
Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets.Signal Transduct Target Ther. 2024 Apr 26;9(1):97. doi: 10.1038/s41392-024-01811-6. Signal Transduct Target Ther. 2024. PMID: 38664391 Free PMC article. Review.
-
AQP3-mediated activation of the AMPK/SIRT1 signaling pathway curtails gallstone formation in mice by inhibiting inflammatory injury of gallbladder mucosal epithelial cells.Mol Med. 2023 Aug 28;29(1):116. doi: 10.1186/s10020-023-00712-8. Mol Med. 2023. PMID: 37641009 Free PMC article.
-
Effects of laparoscopic and choledochoscopic gallbladder-preserving cholecystolithotomy on levels of operation indicators, gallbladder function, and cholecystokinin type-A receptor in patients with gallstones.Wideochir Inne Tech Maloinwazyjne. 2023 Sep;18(3):494-501. doi: 10.5114/wiitm.2023.131076. Epub 2023 Sep 6. Wideochir Inne Tech Maloinwazyjne. 2023. PMID: 37868281 Free PMC article.
References
-
- Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. - PubMed
-
- Fernandez-Rojo MA, Ramm GA. Caveolin-1 Function in Liver Physiology and Disease. Trends Mol Med. 2016;22:889–904. - PubMed
-
- Hernandez-Nazara A, Curiel-Lopez F, Martinez-Lopez E, Hernandez-Nazara Z, Panduro A. Genetic predisposition of cholesterol gallstone disease. Ann Hepatol. 2006;5:140–9. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

