Application of lymphoplasmapheresis in the treatment of severe myasthenia gravis
- PMID: 36303555
- PMCID: PMC9595276
- DOI: 10.3389/fneur.2022.1018509
Application of lymphoplasmapheresis in the treatment of severe myasthenia gravis
Abstract
Background: Lymphoplasmapheresis (LPE) is a treatment that combines traditional plasma exchange and lymphocyte removal technique. It has been applied to treat a variety of autoimmune diseases, but its application value in the treatment of severe myasthenia gravis (MG) is not yet clear. Therefore, the aim of this study was to investigate the efficacy and safety of LPE in severe MG.
Methods: Clinical data of 123 severe patients with MG (Myasthenia Gravis Foundation of America Clinical Classification, Class IV) who received LPE treatment were included in a retrospective analysis. Efficacy was evaluated by the change of Quantitative Myasthenia Gravis score (QMGS) before and after treatment. Univariate and multivariate logistic regression analysis was used to explore clinical factors affecting efficacy.
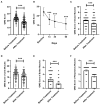
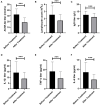
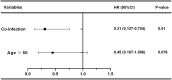
Results: A total of 220 replacements were performed in 123 patients, with an average of 1.79 replacements per patient. The overall safety of LPE was good, and no serious adverse reactions occurred. After treatment, the mean QMGS of patients decreased significantly, from 23.40 ± 4.25 points before treatment to 17.93 ± 5.61 points after treatment, a decrease of 5.47 ± 4.16 points. 75.6% of patients experienced remission of clinical symptoms. During a 2-month follow-up of 64 patients, a progressive improvement in QMGS was found. Each muscle group involved in MG responded well to LPE treatment. In addition, LPE significantly reduced the levels of AChR-Ab and inflammatory cytokines in patients. Age ≥ 50 years and co-infection were unfavorable factors affecting the efficacy.
Conclusions: In this study cohort, LPE is safe for the treatment of severe MG and achieves good treatment outcome with fewer replacements. In patients with MG, the avoidance and timely control of infection are necessary. Our study provides a potential new treatment option for severe MG.
Keywords: efficacy; immunotherapy; lymphoplasmapheresis; myasthenia gravis; safety.
Copyright © 2022 Duan, Zhou, Dong, Li, Li, Cai, Zhou, Ouyang, Yin and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Lymphoplasmapheresis versus plasma exchange in severe myasthenia gravis: a retrospective cohort study.Front Neurol. 2023 Jun 27;14:1212868. doi: 10.3389/fneur.2023.1212868. eCollection 2023. Front Neurol. 2023. PMID: 37441608 Free PMC article.
-
Comparison of Efficacy Between Lymphoplasmapheresis and Plasma Exchange in Myasthenic Crisis.Muscle Nerve. 2025 Jul;72(1):100-106. doi: 10.1002/mus.28422. Epub 2025 Apr 29. Muscle Nerve. 2025. PMID: 40296675
-
Lymphoplasma Exchange Improves Myasthenia Gravis Exacerbations: A Retrospective Study in a Chinese Center.Front Immunol. 2022 Apr 14;13:757841. doi: 10.3389/fimmu.2022.757841. eCollection 2022. Front Immunol. 2022. PMID: 35514988 Free PMC article.
-
Effectiveness and safety of tacrolimus therapy for myasthenia gravis: A single arm meta-analysis.J Clin Neurosci. 2019 May;63:160-167. doi: 10.1016/j.jocn.2019.02.004. Epub 2019 Mar 1. J Clin Neurosci. 2019. PMID: 30827886
-
Is thymectomy in non-thymomatous myasthenia gravis of any benefit?Interact Cardiovasc Thorac Surg. 2014 Mar;18(3):381-9. doi: 10.1093/icvts/ivt510. Epub 2013 Dec 18. Interact Cardiovasc Thorac Surg. 2014. PMID: 24351507 Free PMC article. Review.
Cited by
-
Efgartigimod as a fast-acting treatment in generalized very-late-onset myasthenia gravis.Front Immunol. 2025 Apr 17;16:1579859. doi: 10.3389/fimmu.2025.1579859. eCollection 2025. Front Immunol. 2025. PMID: 40313945 Free PMC article.
-
Continuous blood exchange in rats as a novel approach for experimental investigation.Sci Rep. 2024 May 28;14(1):12194. doi: 10.1038/s41598-024-63049-0. Sci Rep. 2024. PMID: 38806542 Free PMC article.
References
LinkOut - more resources
Full Text Sources

