Novel In Vitro Study to Assess Microbial Barrier Properties of Polyurethane-Based Tissue Adhesives in Comparison to the Gold Standard Dermabond®
- PMID: 36303586
- PMCID: PMC9596255
- DOI: 10.1155/2022/5249214
Novel In Vitro Study to Assess Microbial Barrier Properties of Polyurethane-Based Tissue Adhesives in Comparison to the Gold Standard Dermabond®
Abstract
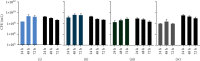
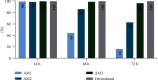
Tissue adhesives as a physical barrier to microorganism penetration provide an alternative method with many advantages for wound closure in surgical settings compared to the clinical standard. This raises the need of developing and conducting in vitro methods that are sensitive and reproducible to assess their microbial barrier properties. In this study, three different polyurethane-based tissue adhesives with different physicochemical properties were evaluated in comparison to Dermabond® as a clinical gold standard for topical wound closure. Here, physicochemical properties varied in lactide concentration, viscosity, processing, and the full polymerization time. To evaluate the microbial barrier function, a 5 μl aliquot of E. coli Lux inoculum containing at least 1 × 109 CFU/ml was applied to the surface of each test adhesive and sterile filter paper as the control that was placed on an agar plate and incubated at 37°C. Plates were observed for bacterial growth (morphology), the adhesion of the adhesive/filter paper, and bioluminescence after 24, 48, and 72 hours. The data presented in this in vitro model indicated that polyurethane-based tissue adhesives with lactide concentration ≥ 5% provided a suitable barrier against microbial penetration with 95% confidence of 99% efficacy for 72 h along with Dermabond®. Interestingly, the here described method was able to discriminate between the different physicochemical properties showing a better microbial barrier function with increasing lactide concentration of the adhesive. Overall, the results of this study showed the noninferiority between Dermabond® and the two abovementioned polyurethane-based tissue adhesives.
Copyright © 2022 Yalda Mirzaei et al.
Conflict of interest statement
Dr. Kerstin Hagemeister was employed at Adhesys Medical GmbH. There are no other conflicts of interest to declare.
Figures


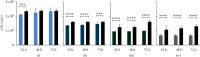
References
-
- Dompé M., Vahdati M., van Ligten F., et al. Enhancement of the adhesive properties by optimizing the water content in PNIPAM-functionalized complex coacervates. ACS Applied Polymer Materials . 2020;2(4):1722–1730. doi: 10.1021/acsapm.0c00185. - DOI
-
- Olympia R. P., O'Neill R., Silvis M. Urgent Care Medicine Secrets E-Book . Elsevier Health Sciences; 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

