Non-Invasive and Mechanism-Based Molecular Assessment of Endometrial Receptivity During the Window of Implantation: Current Concepts and Future Prospective Testing Directions
- PMID: 36303672
- PMCID: PMC9580756
- DOI: 10.3389/frph.2022.863173
Non-Invasive and Mechanism-Based Molecular Assessment of Endometrial Receptivity During the Window of Implantation: Current Concepts and Future Prospective Testing Directions
Abstract
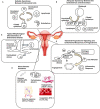
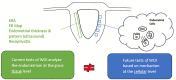
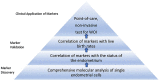
Suboptimal endometrial receptivity and altered embryo-endometrial crosstalk account for approximately two-thirds of human implantation failures. Current tests of the window of implantation, such as endometrial thickness measurements and the endometrial receptivity assay, do not consistently improve clinical outcomes as measured by live birth rates. Understanding the mechanisms regulating the endometrial receptivity during the window of implantation is a critical step toward developing clinically meaningful tests. In this narrative review, the available literature is evaluated regarding mechanisms that regulate the endometrial receptivity during the window of implantation and the current tests developed. Overall, both animal and human studies point to five possible and interrelated mechanisms regulating the endometrial window of implantation: suitable synchrony between endometrial cells, adequate synchrony between the endometrium and the embryo, standard progesterone signaling and endometrial responses to progesterone, silent genetic variations, and typical morphological characteristics of the endometrial glands. The biological basis of current clinical markers or tests of window of implantation is poor. Future studies to elucidate the mechanisms shaping the window of implantation and to investigate the potential markers based on these mechanisms are required. In addition, molecular testing of the endometrium at single-cell resolution should be an initial step toward developing clinically meaningful tests for the optimal window of implantation. As understanding of the optimal window of implantation continues to evolve, one can envision the future development of non-invasive, mechanism-based testing of the window of implantation.
Keywords: endometrial receptivity; mechanism; molecular markers; non-invasive testing; point-of-care testing; window of implantation (WOI).
Copyright © 2022 Sun and Yeh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

