The female reproductive tract microbiotas, inflammation, and gynecological conditions
- PMID: 36303679
- PMCID: PMC9580710
- DOI: 10.3389/frph.2022.963752
The female reproductive tract microbiotas, inflammation, and gynecological conditions
Abstract
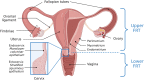
The intricate interactions between the host cells, bacteria, and immune components that reside in the female reproductive tract (FRT) are essential in maintaining reproductive tract homeostasis. Much of our current knowledge surrounding the FRT microbiota relates to the vaginal microbiota, where 'health' has long been associated with low bacterial diversity and Lactobacillus dominance. This concept has recently been challenged as women can have a diverse vaginal microbial composition in the absence of symptomatic disease. The structures of the upper FRT (the endocervix, uterus, Fallopian tubes, and ovaries) have distinct, lower biomass microbiotas than the vagina; however, the existence of permanent microbiotas at these sites is disputed. During homeostasis, a balance exists between the FRT bacteria and the immune system that maintains immune quiescence. Alterations in the bacteria, immune system, or local environment may result in perturbances to the FRT microbiota, defined as dysbiosis. The inflammatory signature of a perturbed or "dysbiotic" FRT microbiota is characterized by elevated concentrations of pro-inflammatory cytokines in cervical and vaginal fluid. It appears that vaginal homeostasis can be disrupted by two different mechanisms: first, a shift toward increased bacterial diversity can trigger vaginal inflammation, and second, local immunity is altered in some manner, which disrupts the microbiota in response to an environmental change. FRT dysbiosis can have negative effects on reproductive health. This review will examine the increasing evidence for the involvement of the FRT microbiotas and inflammation in gynecologic conditions such as endometriosis, infertility, and endometrial and ovarian cancer; however, the precise mechanisms by which bacteria are involved in these conditions remains speculative at present. While only in their infancy, the use of antibiotics and probiotics to therapeutically alter the FRT microbiota is being studied and is discussed herein. Our current understanding of the intimate relationship between immunity and the FRT microbiota is in its early days, and more research is needed to deepen our mechanistic understanding of this relationship and to assess how our present knowledge can be harnessed to assist in diagnosis and treatment of gynecologic conditions.
Keywords: female reproductive tract (FRT); gynecological diseases; immune response; inflammation; microbiome.
Copyright © 2022 Gholiof, Adamson-De Luca and Wessels.
Conflict of interest statement
Authors JW is Co-founder and Chief Scientific Officer and EA-D is a Product Manager at AIMA Laboratories Inc., a company with products designed to address non-malignant gynecological issues in Women's Health. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

