Insights for applying N,S-doped carbon dots as a fluorescent nanoprobe for estimation of some nitro-calcium channel blockers
- PMID: 36303941
- PMCID: PMC9597176
- DOI: 10.1098/rsos.220609
Insights for applying N,S-doped carbon dots as a fluorescent nanoprobe for estimation of some nitro-calcium channel blockers
Abstract
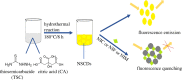
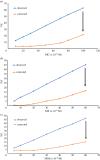
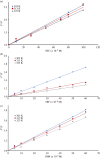
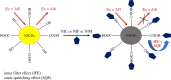
A facile and simple one-step hydrothermal approach was adopted for fabrication of N and S co-doped carbon quantum dots probe (NSCDs) by using thiosemicarbazide as a dopant and citric acid as a precursor. The prepared NSCDs with a high quantum yield of 0.58 were characterized using UV-visible spectroscopy, IR spectroscopy and high-resolution transmission electron microscopy. The as-obtained NSCDs could be deemed as an effective fluorescent nanosensor for the determination of some anti-hypertensive nitro-calcium channel blockers (Nitro-CCBs) including nicardipine (NIC), nifedipine (NIF) and nimodipine (NIM) whether in pure form or in their pharmaceutical formulations. Measurements of NSCD emission intensity were performed at 416 nm after being excited at 345 nm. Nitro-CCBs could induce quenching in the native fluorescence of NSCDs due to the inner filter effect and static quenching mechanism. The studied compounds were investigated within linear detection range of (10.0-100.0 µM) for NIC, (5.0-60.0 µM) for NIF and (5.0-60.0 µM) for NIM. Correlation coefficients are greater than or equal to 0.9998 and detection limits are ranged between 0.55 and 1.86 µM. The proposed method was extended to estimate the studied compounds in different pharmaceutical samples with high % recoveries ranging from (97.95 to 101.28%) and low % relative standard deviation values (less than 2%). Validation of the developed spectrofluorimetric method was done along with the International Council of Harmonization requirements.
Keywords: inner filter effect; nicardipine; nifedipine; nimodipine; nitrogen/sulfur carbon quantum dots.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Application of sulfur and nitrogen doped carbon quantum dots as sensitive fluorescent nanosensors for the determination of saxagliptin and gliclazide.R Soc Open Sci. 2022 Jun 1;9(6):220285. doi: 10.1098/rsos.220285. eCollection 2022 Jun. R Soc Open Sci. 2022. PMID: 35706663 Free PMC article.
-
Fluorescent nitrogen and sulfur co-doped carbon dots from casein and their applications for sensitive detection of Hg2+ and biothiols and cellular imaging.Anal Chim Acta. 2017 Apr 29;964:150-160. doi: 10.1016/j.aca.2017.01.037. Epub 2017 Jan 31. Anal Chim Acta. 2017. PMID: 28351631
-
Turn-off fluorescence of S,N-doped carbon dots for determination of two nitro-containing drugs in dosage forms and human plasma.Spectrochim Acta A Mol Biomol Spectrosc. 2023 Mar 15;289:122246. doi: 10.1016/j.saa.2022.122246. Epub 2022 Dec 15. Spectrochim Acta A Mol Biomol Spectrosc. 2023. PMID: 36542924
-
Utilization of N,S-doped carbon dots as a fluorescent nanosensor for determination of cromolyn based on inner filter effect: application to aqueous humour.Luminescence. 2022 May;37(5):713-721. doi: 10.1002/bio.4212. Epub 2022 Feb 28. Luminescence. 2022. PMID: 35158415
-
A Design-assisted Spectrofluorometric Method Utilizing a One-pot Fluorescent Probe for the Quantitation of some Calcium Channel Blockers.J Fluoresc. 2023 Mar;33(2):671-683. doi: 10.1007/s10895-022-03089-9. Epub 2022 Dec 8. J Fluoresc. 2023. PMID: 36480125 Free PMC article.
Cited by
-
Sensitive Detection of Copper (II) Ions in Aqueous Solutions Using Thioglycolic Acid-Capped Ce-Doped ZnS Nanoparticles Based Fluorescent Probe.J Fluoresc. 2025 Jun 21. doi: 10.1007/s10895-025-04335-6. Online ahead of print. J Fluoresc. 2025. PMID: 40542984
References
-
- Godfraind T. 2004. Calcium channel blockers. Berlin, Germany: Springer Science & Business Media.
-
- Sweetman S. 2009. Martindale: the complete drug reference, 36th edn. London, UK: Pharmaceutical Press.
-
- Moffat AC, Osselton MD, Widdop B, Galichet LY. 2011. Clark's analysis of drugs and poisons in pharmaceuticals, body fluids and postmortem material, vol. II, 4th edn, pp. 1777-1781. London, UK: Pharmaceutical Press.
-
- Derayea SM, Askal HF, Abdel-Megeed OH, El Hamd MA. 2012. Spectrophotometric determination of amlodipine and nicardipine in pharmaceutical formulations via binary complex formation with eosin Y. J. Appl. Pharm. Sci. 2, 84-89. (10.7324/JAPS.2012.2622) - DOI
-
- Hamd M, Abdellatif A, Derayea S, Abdelmageed O, Askal H. 2015. Spectrophotometric determination of nifedipine and nicardipine in their pharmaceutical preparations. Ind. Chem. 1, 103. (10.4172/2471-2663.1000103) - DOI
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

