Prevalence of Human Papillomavirus (HPV) Genotypes in Cervicovaginal Secretions of Human Immunodeficiency Virus (HIV) Positive Indian Women and Correlation With Clinico-Virological Parameters
- PMID: 36303978
- PMCID: PMC9580721
- DOI: 10.3389/frph.2021.695254
Prevalence of Human Papillomavirus (HPV) Genotypes in Cervicovaginal Secretions of Human Immunodeficiency Virus (HIV) Positive Indian Women and Correlation With Clinico-Virological Parameters
Abstract
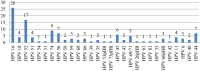
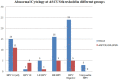
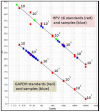
Introduction and Background: Both human papillomavirus (HPV) and the human immunodeficiency virus (HIV) are sexually transmitted. High-risk (HR) HPV types are a causal factor in cervical cancer. Persistent HPV infection in this subset of immunocompromised women results in faster disease progression. The study determined the prevalence of HPV genotypes in cervicovaginal secretions of HIV seropositive women and the correlation with CD4 counts and cytology. Method: One hundred, non-pregnant, HIV-positive women of 18 years of age and above were enrolled in this cross-sectional study following approval by the institutional ethical committee. A written consent, questionnaire, followed by sample collection including a Papanicolaou (Pap) smear for cytology was undertaken. Cervicovaginal secretion samples were collected in the Digene® specimen transport medium (STM) (Qiagen Gaithersburg Inc., MD, USA). HPV genotyping was carried out with PCR amplification of a 65-base pair (bp) fragment in the L1 region of the HPV genome using the short PCR fragment (SPF10) primers followed by reverse hybridization by line probe assay (LPA) using the INNOLiPA HPV Genotyping Extra kit (Fujirebio, Belgium). Quantitation of HPV-16 and-18 viral loads (VLs) was done by real-time PCR. Results of Pap smear cytology were correlated with CD4 counts and HPV-16 and-18 VLs. Results: Mean age of the subjects was 34.9 years ± 7.2 years (median 33.0 years, range 24-60 years). HPV was detected in 62 of 93 (66.6%) samples. Twenty (32.25%) of these 62 samples harbored a single HPV genotype. Multiple genotypes (more than two) were detected in 38 (61.3%) samples. HPV-16 was the commonest genotype detected in 26 (27.9%) of all samples and 41.9% of HPV positive samples. Pap smear cytology was reported for 93 women included in the study. Women who had normal cytology were reported as negative for intraepithelial malignancy or lesion (NILM; n = 62; 71.36%), two women had a high-grade squamous intraepithelial lesion (HSIL), low-grade squamous intraepithelial lesion (LSIL; n = 11), atypical squamous cells of undetermined significance (ASCUS; n = 12). Those smears with inadequate material were reported as scant (n = 6). The median CD4 count was 363/cu.mm (range 39-787) in HPV-positive women compared to 423/cu.mm (range 141-996) in those HPV-negative women. Quantitation of HPV-16 and-18 VL was done in duplicate for samples positive by PCR reverse hybridization (INNOLiPA). Of these 20 samples (65%), 12 samples were positive by real-time PCR. The normalized HPV-16 VL ranged between 18 and 240,000 copies/cell. The normalized HPV-18 VL in cervical samples ranged between ~24 and 60,000 copies/cell. Conclusion: HIV-positive women may be infected with multiple genotypes other than HPV-16 and-18. This may have implications on the vaccines available currently which target few specific genotypes only. Studies are required to determine the predictive role of HR HPV genotypes, in significant copy numbers especially in HIV seropositive women. It would be clinically relevant if the HPV VLs, cervical cytology, and CD4 counts are considered into cervical cancer screening programs for triage and follow-up of these women.
Keywords: HIV; HPV; cervical cancer; clinicopathological correlation; cytology; genotypes.
Copyright © 2021 Lall, Dar, Bhatla, Kumar, Choudhary, Mathur and Gupta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The prevelance of human papillomavirus (HPV) genotypes detected by PCR in women with normal and abnormal cervico-vaginal cytology.Ginekol Pol. 2018;89(2):62-67. doi: 10.5603/GP.a2018.0011. Ginekol Pol. 2018. PMID: 29512809
-
Molecular Pap smear: HPV genotype and DNA methylation of ADCY8, CDH8, and ZNF582 as an integrated biomarker for high-grade cervical cytology.Clin Epigenetics. 2016 Sep 13;8(1):96. doi: 10.1186/s13148-016-0263-9. eCollection 2016. Clin Epigenetics. 2016. PMID: 27651839 Free PMC article.
-
Prevalence of human papillomavirus infection & cervical abnormalities in HIV-positive women in eastern India.Indian J Med Res. 2016 Jan;143(1):79-86. doi: 10.4103/0971-5916.178614. Indian J Med Res. 2016. PMID: 26997018 Free PMC article.
-
Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer.Vaccine. 2012 Nov 20;30 Suppl 5:F88-99. doi: 10.1016/j.vaccine.2012.06.095. Vaccine. 2012. PMID: 23199969 Review.
-
Prevalence and distribution of human papillomavirus genotypes in women with abnormal cervical cytology in Ethiopia: a systematic review and meta-analysis.Front Oncol. 2024 Oct 15;14:1384994. doi: 10.3389/fonc.2024.1384994. eCollection 2024. Front Oncol. 2024. PMID: 39474105 Free PMC article.
Cited by
-
The impact of HPV/HIV co-infection on immunosuppression, HPV genotype, and cervical cancer biomarkers.BMC Cancer. 2025 Feb 5;25(1):202. doi: 10.1186/s12885-025-13516-2. BMC Cancer. 2025. PMID: 39910495 Free PMC article.
References
-
- Global Strategy . Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Geneva: World Health Organization; (2020).
-
- Kelly HA, Chikandiwa A, Sawadogo B, Gilham C, Michelow P, Lompo OG, et al. Diagnostic accuracy of cervical cancer screening and screening–triage strategies among women living with HIV-1 in Burkina Faso and South Africa: A cohort study. PLoS Med. (2021) 18:e1003528. 10.1371/journal.pmed.1003528 - DOI - PMC - PubMed
-
- International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100 B, Biological Agents: IARC; (2012). p. 475.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

