Exploring metabolism in scleroderma reveals opportunities for pharmacological intervention for therapy in fibrosis
- PMID: 36304460
- PMCID: PMC9592691
- DOI: 10.3389/fimmu.2022.1004949
Exploring metabolism in scleroderma reveals opportunities for pharmacological intervention for therapy in fibrosis
Abstract
Background: Recent evidence has indicated that alterations in energy metabolism play a critical role in the pathogenesis of fibrotic diseases. Studies have suggested that 'metabolic reprogramming' involving the glycolysis and oxidative phosphorylation (OXPHOS) in cells lead to an enhanced generation of energy and biosynthesis. The aim of this study was to assess the molecular basis of changes in fibrotic metabolism in systemic sclerosis (Scleroderma; SSc) and highlight the most appropriate targets for anti-fibrotic therapies.
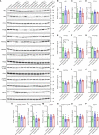
Materials and methods: Dermal fibroblasts were isolated from five SSc patients and five healthy donors. Cells were cultured in medium with/without TGF-β1 and with/without ALK5, pan-PIM or ATM kinase inhibitors. Extracellular flux analyses were performed to evaluate glycolytic and mitochondrial respiratory function. The mitochondrial network in TMRM-stained cells was visualized by confocal laser-scanning microscopy, followed by semi-automatic analysis on the ImageJ platform. Protein expression of ECM and fibroblast components, glycolytic enzymes, subunits of the five OXPHOS complexes, and dynamin-related GTPases and receptors involved in mitochondrial fission/fusion were assessed by western blotting.
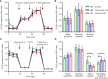
Results: Enhanced mitochondrial respiration coupled to ATP production was observed in SSc fibroblasts at the expense of spare respiratory capacity. Although no difference was found in glycolysis when comparing SSc with healthy control fibroblasts, levels of phophofructokinase-1 isoform PFKM were significantly lower in SSc fibroblasts (P<0.05). Our results suggest that the number of respirasomes is decreased in the SSc mitochondria; however, the organelles formed a hyperfused network, which is thought to increase mitochondrial ATP production through complementation. The increased mitochondrial fusion correlated with a change in expression levels of regulators of mitochondrial morphology, including decreased levels of DRP1, increased levels of MIEF2 and changes in OPA1 isoform ratios. TGF-β1 treatment strongly stimulated glycolysis and mitochondrial respiration and induced the expression of fibrotic markers. The pan-PIM kinase inhibitor had no effect, whereas both ALK5 and ATM kinase inhibition abrogated TGF-β1-mediated fibroblast activation, and upregulation of glycolysis and respiration.
Conclusions: Our data provide evidence for a novel mechanism(s) by which SSc fibroblasts exhibit altered metabolic programs and highlight changes in respiration and dysregulated mitochondrial morphology and function, which can be selectively targeted by small molecule kinase inhibitors.
Keywords: fibrosis; glycolysis; kinase inhibitors; mitochondrial morphology; mitochondrial respiration; myofibroblasts; oxidative phosphorylation; systemic sclerosis.
Copyright © 2022 Cantanhede, Liu, Liu, Balbuena Rodriguez, Shiwen, Ong, Denton, Ponticos, Xiong, Lima-Filho, Abraham, Abu-Hanna and Taanman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Metabolic reprogramming of glycolysis and glutamine metabolism are key events in myofibroblast transition in systemic sclerosis pathogenesis.J Cell Mol Med. 2020 Dec;24(23):14026-14038. doi: 10.1111/jcmm.16013. Epub 2020 Nov 2. J Cell Mol Med. 2020. PMID: 33140521 Free PMC article.
-
Extracellular SPARC cooperates with TGF-β signalling to induce pro-fibrotic activation of systemic sclerosis patient dermal fibroblasts.Rheumatology (Oxford). 2020 Sep 1;59(9):2258-2263. doi: 10.1093/rheumatology/kez583. Rheumatology (Oxford). 2020. PMID: 31840182 Free PMC article.
-
[Expression and significance of ferroptosis marker 4-HNE in in vitro model of systemic sclerosis].Beijing Da Xue Xue Bao Yi Xue Ban. 2024 Dec 18;56(6):950-955. doi: 10.19723/j.issn.1671-167X.2024.06.002. Beijing Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39690754 Free PMC article. Chinese.
-
Pathogenesis of scleroderma. Collagen.Rheum Dis Clin North Am. 1996 Nov;22(4):647-74. doi: 10.1016/s0889-857x(05)70294-5. Rheum Dis Clin North Am. 1996. PMID: 8923589 Review.
-
The role of metabolism in the pathogenesis of systemic sclerosis.Metabolism. 2019 Apr;93:44-51. doi: 10.1016/j.metabol.2018.12.004. Epub 2018 Dec 23. Metabolism. 2019. PMID: 30586574 Review.
Cited by
-
Causal association of basal metabolic rate on systemic sclerosis: a bidirectional mendelian randomization study.Arch Dermatol Res. 2024 Aug 22;316(8):553. doi: 10.1007/s00403-024-03248-x. Arch Dermatol Res. 2024. PMID: 39172247
-
Relationships between systemic sclerosis and atherosclerosis: screening for mitochondria-related biomarkers.Front Genet. 2024 Jul 10;15:1375331. doi: 10.3389/fgene.2024.1375331. eCollection 2024. Front Genet. 2024. PMID: 39050259 Free PMC article.
-
Identification and validation of biomarkers related to ferroptosis in idiopathic pulmonary fibrosis.Sci Rep. 2025 Mar 13;15(1):8622. doi: 10.1038/s41598-025-93217-9. Sci Rep. 2025. PMID: 40075162 Free PMC article.
-
Altered AP-1, RUNX and EGR chromatin dynamics drive fibrotic lung disease.bioRxiv [Preprint]. 2024 Oct 28:2024.10.23.619858. doi: 10.1101/2024.10.23.619858. bioRxiv. 2024. PMID: 39554071 Free PMC article. Preprint.
-
Small-scale protocols to characterize mitochondrial Complex V activity and assembly in peripheral blood mononuclear cells.PLoS One. 2025 May 8;20(5):e0323136. doi: 10.1371/journal.pone.0323136. eCollection 2025. PLoS One. 2025. PMID: 40338882 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

