Menstrual Effluent Provides a Novel Diagnostic Window on the Pathogenesis of Endometriosis
- PMID: 36304708
- PMCID: PMC9580670
- DOI: 10.3389/frph.2020.00003
Menstrual Effluent Provides a Novel Diagnostic Window on the Pathogenesis of Endometriosis
Abstract
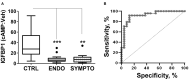
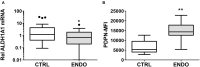
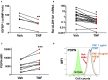
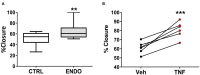
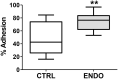
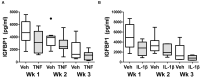
Endometriosis is a chronic inflammatory disorder characterized by the presence of endometrial-like tissue growing outside of the uterus. Although the cause is unknown, retrograde menstruation leads to deposition of endometrial cells into the peritoneal cavity. Lack of disease recognition and long diagnostic delays (6-10 years) lead to substantial personal, social and financial burdens, as well as delayed treatment. A non-invasive diagnostic for endometriosis is a major unmet clinical need. Here, we assessed whether differences in menstrual effluent-derived stromal fibroblast cells (ME-SFCs) from women with and without endometriosis provide the basis for a non-invasive diagnostic for endometriosis. In addition, we investigated whether treatment of control ME-SFCs with inflammatory cytokines (TNF and IL-1β) could induce an endometriosis-like phenotype. ME-SFCs from laparoscopically diagnosed endometriosis patients exhibit reduced decidualization capacity, measured by IGFBP1 production after exposure to cAMP. A receiver operating characteristic (ROC) curve developed using decidualization data from controls and endometriosis subjects yielded an area under the curve of 0.92. In addition, a significant reduction in ALDH1A1 gene expression and increased podoplanin surface expression were also observed in endometriosis ME-SFCs when compared to control ME-SFCs. These endometriosis-like phenotypes can be reproduced in control ME-SFCs by exposure to inflammatory cytokines (TNF and IL-1β) and are associated with increased cell migration. These results are consistent with the hypothesis that chronic intrauterine inflammation influences the development of endometriosis lesions following retrograde menstruation. In conclusion, the analysis of ME-SFCs can provide an accurate, rapid, and non-invasive diagnostic for endometriosis and insight into disease pathogenesis.
Keywords: ALDH1A1; endometriosis; endometrium; inflammatory cytokines; non-invasive diagnostic; podoplanin; stromal fibroblast cells.
Copyright © 2020 Nayyar, Saleem, Yilmaz, DeFranco, Klein, Elmaliki, Kowalsky, Chatterjee, Xue, Viswanathan, Shih, Gregersen and Metz.
Figures
Similar articles
-
Analysis of menstrual effluent: diagnostic potential for endometriosis.Mol Med. 2018 Mar 19;24(1):1. doi: 10.1186/s10020-018-0009-6. Mol Med. 2018. PMID: 30134794 Free PMC article.
-
Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis.BMC Med. 2022 Sep 15;20(1):315. doi: 10.1186/s12916-022-02500-3. BMC Med. 2022. PMID: 36104692 Free PMC article.
-
Endometrial inflammasome activation accompanies menstruation and may have implications for systemic inflammatory events of the menstrual cycle.Hum Reprod. 2020 Jun 1;35(6):1363-1376. doi: 10.1093/humrep/deaa065. Hum Reprod. 2020. PMID: 32488243
-
[Endometriosis].Rev Med Univ Navarra. 2009 Apr-Jun;53(2):4-7. Rev Med Univ Navarra. 2009. PMID: 19994762 Review. Spanish.
-
The role of endometrium in endometriosis.J Soc Gynecol Investig. 2006 Oct;13(7):467-76. doi: 10.1016/j.jsgi.2006.07.005. Epub 2006 Sep 20. J Soc Gynecol Investig. 2006. PMID: 16990031 Review.
Cited by
-
Progesterone-induced progesterone receptor membrane component 1 rise-to-decline changes are essential for decidualization.Reprod Biol Endocrinol. 2024 Feb 3;22(1):20. doi: 10.1186/s12958-024-01188-9. Reprod Biol Endocrinol. 2024. PMID: 38308254 Free PMC article.
-
Endometriosis and Cardiovascular Disease: Exploring Pathophysiological Interconnections and Risk Mechanisms.Diagnostics (Basel). 2025 Jun 8;15(12):1458. doi: 10.3390/diagnostics15121458. Diagnostics (Basel). 2025. PMID: 40564779 Free PMC article. Review.
-
Inflammatory Mechanisms of Dysmenorrhea: Novel Insights From Menstrual Effluent in an Adolescent Cohort.BJOG. 2025 Jul 9:10.1111/1471-0528.18275. doi: 10.1111/1471-0528.18275. Online ahead of print. BJOG. 2025. PMID: 40631483
-
Targeting Cellular Senescence to Enhance Human Endometrial Stromal Cell Decidualization and Inhibit Their Migration.Biomolecules. 2025 Jun 16;15(6):873. doi: 10.3390/biom15060873. Biomolecules. 2025. PMID: 40563513 Free PMC article.
-
Quercetin enhances decidualization through AKT-ERK-p53 signaling and supports a role for senescence in endometriosis.Reprod Biol Endocrinol. 2024 Aug 8;22(1):100. doi: 10.1186/s12958-024-01265-z. Reprod Biol Endocrinol. 2024. PMID: 39118090 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

