Hydrophobic Gold Nanoparticles with Intrinsic Chirality for the Efficient Fabrication of Chiral Plasmonic Nanocomposites
- PMID: 36305423
- PMCID: PMC9650650
- DOI: 10.1021/acsami.2c11925
Hydrophobic Gold Nanoparticles with Intrinsic Chirality for the Efficient Fabrication of Chiral Plasmonic Nanocomposites
Abstract
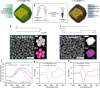
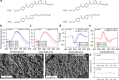
The development of plasmonic nanomaterials with chiral geometry has drawn extensive attention owing to their practical implications in chiral catalysis, chiral metamaterials, or enantioselective biosensing and medicine. However, due to the lack of effective synthesis methods of hydrophobic nanoparticles (NPs) showing intrinsic, plasmonic chirality, their applications are currently limited to aqueous systems. In this work, we resolve the problem of achieving hydrophobic Au NPs with intrinsic chirality by efficient phase transfer of water-soluble NPs using low molecular weight, liquid crystal-like ligands. We confirmed that, after the phase transfer, Au NPs preserve strong, far-field circular dichroism (CD) signals, attesting their chiral geometry. The universality of the method is exemplified by using different types of NPs and ligands. We further highlight the potential of the proposed approach to realize chiral plasmonic, inorganic/organic nanocomposites with block copolymers, liquid crystals, and compounds forming physical gels. All soft matter composites sustain plasmonic CD signals with electron microscopies confirming well-dispersed nanoinclusions. The developed methodology allows us to expand the portfolio of plasmonic NPs with intrinsic structural chirality, thereby broadening the scope of their applications toward soft-matter based systems.
Keywords: block copolymers; chiral metamolecules; chirality transfer; circular dichroism; gels; liquid crystals; phase transfer; reconfigurable nanostructures; supramolecular chirality.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Multidentate Chirality Inducers in the Synthesis of Intrinsically Chiral Gold Nanoparticles: Effects of Multiplicity and Spatial Distribution of Cysteine.Small. 2025 Jul 16:e2408004. doi: 10.1002/smll.202408004. Online ahead of print. Small. 2025. PMID: 40665868
-
Light-to-matter chirality transfer in plasmonics.Mater Horiz. 2025 Jul 14;12(14):4940-4969. doi: 10.1039/d5mh00179j. Mater Horiz. 2025. PMID: 40397475 Review.
-
Polymer-Driven Co-Assembly of Achiral and Chiral Nanoparticles into Plasmonic Nanoclusters with Quantitatively Modulated Optical Chirality.Adv Sci (Weinh). 2025 Jun 19:e04850. doi: 10.1002/advs.202504850. Online ahead of print. Adv Sci (Weinh). 2025. PMID: 40536314
-
Geometric Control and Optical Properties of Intrinsically Chiral Plasmonic Nanomaterials.Adv Mater. 2025 Aug;37(31):e2306297. doi: 10.1002/adma.202306297. Epub 2023 Nov 29. Adv Mater. 2025. PMID: 37572380 Review.
-
Chiral Cobaltabis(dicarbollide) Catalysts Prepared Using a Silica Helical Nanoplatform for the Enantioselective Photooxidation of Aromatic Secondary Alcohols.Chemistry. 2025 Jul 2;31(37):e202501213. doi: 10.1002/chem.202501213. Epub 2025 May 19. Chemistry. 2025. PMID: 40333997 Free PMC article.
Cited by
-
Engineering copper plasmonic chirality via ligand-induced dissolution for enantioselective recognition of amino acids.Chem Sci. 2024 Apr 16;15(19):7121-7129. doi: 10.1039/d4sc00477a. eCollection 2024 May 15. Chem Sci. 2024. PMID: 38756802 Free PMC article.
-
Synthesis and Characterization of Gold Chiral Nanoparticles Functionalized by a Chiral Drug.Nanomaterials (Basel). 2023 Apr 30;13(9):1526. doi: 10.3390/nano13091526. Nanomaterials (Basel). 2023. PMID: 37177071 Free PMC article.
-
Biomimetic Chiral Nanomaterials with Selective Catalysis Activity.Adv Sci (Weinh). 2024 Jun;11(23):e2306979. doi: 10.1002/advs.202306979. Epub 2024 Apr 1. Adv Sci (Weinh). 2024. PMID: 38561968 Free PMC article. Review.
-
A single nanophotonic platform for producing circularly polarized white light from non-chiral emitters.Nat Commun. 2024 Nov 30;15(1):10443. doi: 10.1038/s41467-024-54792-z. Nat Commun. 2024. PMID: 39616193 Free PMC article.
References
-
- Wang L.; Hasanzadeh Kafshgari M.; Meunier M. Optical Properties and Applications of Plasmonic-Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400.10.1002/adfm.202005400. - DOI
-
- Zhao X.; Yang L.; Guo J.; Xiao T.; Zhou Y.; Zhang Y.; Tu B.; Li T.; Grzybowski B. A.; Yan Y. Transistors and Logic Circuits Based on Metal Nanoparticles and Ionic Gradients. Nat. Electron. 2021, 4 (2), 109–115. 10.1038/s41928-020-00527-z. - DOI
LinkOut - more resources
Full Text Sources

