Downregulation of iNOS/NO Promotes Epithelial-Mesenchymal Transition and Metastasis in Colorectal Cancer
- PMID: 36306210
- PMCID: PMC9890133
- DOI: 10.1158/1541-7786.MCR-22-0509
Downregulation of iNOS/NO Promotes Epithelial-Mesenchymal Transition and Metastasis in Colorectal Cancer
Abstract
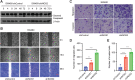
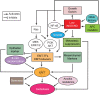
Metastasis is the major cause of cancer-related death in patients with colorectal cancer. Although inducible nitric oxide synthase (iNOS) is a crucial regulator of cancer development and progression, its roles in epithelial-mesenchymal transition (EMT) and the pathogenesis of metastatic colorectal cancer have not been fully investigated. Primary colorectal cancer and liver metastatic tissue specimens were analyzed showing 90% of liver metastatic colorectal cancer with reduced expressions of iNOS compared with 6% of primary colorectal cancer. The Cancer Genome Atlas database analyses via cBioPortal reveal that mRNA expression of iNOS negatively correlated with selected EMT markers in colorectal cancer in a cancer type-dependent manner. The transcriptomic profiling (RNA sequencing data) indicates that iNOS knockdown in SW480 colorectal cancer cells induced an EMT program with upregulated expression of selected stem-cell markers. iNOS knockdown did not alter E-cadherin mRNA expression but re-localized it from membrane to cytoplasm through iNOS-GATA4-Crb2-E-cadherin pathway. iNOS knockdown induced a change in cell morphology, and promoted cell invasion and migration in vitro, and metastasis in vivo.
Implications: iNOS downregulation-induced pathway networks mediate the EMT program and metastasis. As an EMT inducer, the reduced-iNOS may serve as a potential therapeutic target for patients with colorectal cancer.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures





Comment in
- 1541-7786. doi: 10.1158/1541-7786.MCR-21-2-HI
Similar articles
-
NUBPL, a novel metastasis-related gene, promotes colorectal carcinoma cell motility by inducing epithelial-mesenchymal transition.Cancer Sci. 2017 Jun;108(6):1169-1176. doi: 10.1111/cas.13243. Epub 2017 Jun 14. Cancer Sci. 2017. PMID: 28346728 Free PMC article.
-
MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis.Gut. 2013 Sep;62(9):1315-26. doi: 10.1136/gutjnl-2011-301846. Epub 2012 Jun 26. Gut. 2013. PMID: 22735571 Free PMC article.
-
miR-196a-5p promotes metastasis of colorectal cancer via targeting IκBα.BMC Cancer. 2019 Jan 8;19(1):30. doi: 10.1186/s12885-018-5245-1. BMC Cancer. 2019. PMID: 30621631 Free PMC article.
-
MiR-150 alleviates EMT and cell invasion of colorectal cancer through targeting Gli1.Eur Rev Med Pharmacol Sci. 2017 Nov;21(21):4853-4859. Eur Rev Med Pharmacol Sci. 2017. Retraction in: Eur Rev Med Pharmacol Sci. 2020 Jul;24(14):7544. doi: 10.26355/eurrev_202007_22181. PMID: 29164577 Retracted.
-
Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition.J Biol Chem. 2012 Feb 17;287(8):5819-32. doi: 10.1074/jbc.M111.295964. Epub 2011 Dec 28. J Biol Chem. 2012. PMID: 22205702 Free PMC article.
Cited by
-
Clonorchis sinensis legumain promotes migration and invasion of cholangiocarcinoma cells via regulating tumor-related molecules.Parasit Vectors. 2023 Feb 16;16(1):71. doi: 10.1186/s13071-023-05694-4. Parasit Vectors. 2023. PMID: 36797792 Free PMC article.
-
Arginase-1 promotes lens epithelial-to-mesenchymal transition in different models of anterior subcapsular cataract.Cell Commun Signal. 2023 Sep 18;21(1):236. doi: 10.1186/s12964-023-01210-4. Cell Commun Signal. 2023. PMID: 37723490 Free PMC article.
-
Elevated ADH5 expression suggested better prognosis in kidney renal clear cell carcinoma (KIRC) and related to immunity through single-cell and bulk RNA-sequencing.BMC Urol. 2024 Apr 10;24(1):84. doi: 10.1186/s12894-024-01478-9. BMC Urol. 2024. PMID: 38600527 Free PMC article.
-
iNOS-Produced Nitric Oxide from Cancer Cells as an Intermediate of Stemness Regulation by PARP-1 in Colorectal Cancer.Biomolecules. 2025 Jan 14;15(1):125. doi: 10.3390/biom15010125. Biomolecules. 2025. PMID: 39858519 Free PMC article.
-
NOS2 as a prognostic biomarker for early-onset colorectal cancer based on public data and clinical validation analysis.Sci Rep. 2025 Feb 4;15(1):4300. doi: 10.1038/s41598-025-88966-6. Sci Rep. 2025. PMID: 39905237 Free PMC article.
References
-
- Lambert AW, Weinberg RA. Linking EMT programs to normal and neoplastic epithelial stem cells. Nat Rev Cancer 2021;21:325–38. - PubMed
-
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019;20:69–84. - PubMed
-
- de Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13:97–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

