CD11c regulates neutrophil maturation
- PMID: 36306384
- PMCID: PMC10119615
- DOI: 10.1182/bloodadvances.2022007719
CD11c regulates neutrophil maturation
Abstract
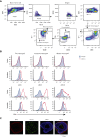
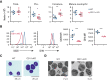
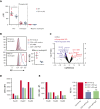
Sepsis continues to be associated with high morbidity and mortality. Currently, sepsis is managed only conservatively. In sepsis, a substantial number of neutrophils is required, leading to accelerated neutrophil production. Immature neutrophils are released into the circulation to meet a demand, despite their less effective functioning in microbial eradication. Although an intervention to provide more mature neutrophils may serve as a potential sepsis treatment, the mechanism of neutrophil differentiation and maturation remains poorly understood. We discovered that CD11c, traditionally known as a dendritic cell marker, was expressed in neutrophils and regulated neutrophil maturation and effector functions. In the absence of CD11c, neutrophil maturation was impaired in the bone marrow, concomitant with a significant increase in the proliferation and apoptosis of preneutrophils, associated with less effector functions. Under lipopolysaccharide challenge, inducing an emergent neutrophil production in the bone marrow, CD11c deficiency exaggerated the release of immature neutrophils into the circulation, associated with a significant proliferation and apoptosis of preneutrophils. In contrast, constitutively active CD11c knock-in mice showed accelerated neutrophil maturation associated with enhanced effector functions, which further supports the notion that CD11c regulates neutrophil maturation. Furthermore, the constitutively active CD11c knock-in mice offered enhanced bacterial eradication. Taken together, we discovered that CD11c was critical for the regulation of neutrophil maturation, and CD11c activation could serve as a potential target for sepsis treatment.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Similar articles
-
Central role of conventional dendritic cells in regulation of bone marrow release and survival of neutrophils.J Immunol. 2014 Apr 1;192(7):3374-82. doi: 10.4049/jimmunol.1300237. Epub 2014 Mar 3. J Immunol. 2014. PMID: 24591364 Free PMC article.
-
The Impact of TRAIL on the Immunological Milieu during the Early Stage of Abdominal Sepsis.Cancers (Basel). 2023 Mar 15;15(6):1773. doi: 10.3390/cancers15061773. Cancers (Basel). 2023. PMID: 36980658 Free PMC article.
-
Mechanisms of neutropenia involving myeloid maturation arrest in burn sepsis.Ann Surg. 1998 Jul;228(1):112-22. doi: 10.1097/00000658-199807000-00017. Ann Surg. 1998. PMID: 9671075 Free PMC article.
-
Dysregulation of neutrophil death in sepsis.Front Immunol. 2022 Aug 18;13:963955. doi: 10.3389/fimmu.2022.963955. eCollection 2022. Front Immunol. 2022. PMID: 36059483 Free PMC article. Review.
-
Modulating neutrophil apoptosis.Novartis Found Symp. 2007;280:53-66; discussion 67-72, 160-4. Novartis Found Symp. 2007. PMID: 17380788 Review.
Cited by
-
Candida albicans aspartyl protease (Sap6) inhibits neutrophil function via a "Trojan horse" mechanism.Sci Rep. 2025 Feb 26;15(1):6946. doi: 10.1038/s41598-025-91425-x. Sci Rep. 2025. PMID: 40011643 Free PMC article.
-
CD11a regulates hematopoietic stem and progenitor cells.Front Immunol. 2023 Sep 14;14:1219953. doi: 10.3389/fimmu.2023.1219953. eCollection 2023. Front Immunol. 2023. PMID: 37781399 Free PMC article.
-
Ocular surface immune transcriptome and tear cytokines in corneal infection patients.Front Cell Infect Microbiol. 2024 Apr 17;14:1346821. doi: 10.3389/fcimb.2024.1346821. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38694515 Free PMC article.
-
Multifaceted, unique role of CD11c in leukocyte biology.Front Immunol. 2025 Mar 4;16:1556992. doi: 10.3389/fimmu.2025.1556992. eCollection 2025. Front Immunol. 2025. PMID: 40103815 Free PMC article. Review.
-
Accelerating Oral Wound Healing Using Bilayer Biomaterial Delivery of FTY720 Immunotherapy.Adv Healthc Mater. 2024 Dec;13(30):e2401480. doi: 10.1002/adhm.202401480. Epub 2024 Oct 10. Adv Healthc Mater. 2024. PMID: 39388502 Free PMC article.
References
-
- Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873. - PubMed
-
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. - PubMed
-
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. - PubMed
-
- Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

