The impact of in utero transfusions on perinatal outcomes in patients with alpha thalassemia major: the UCSF registry
- PMID: 36306387
- PMCID: PMC9860434
- DOI: 10.1182/bloodadvances.2022007823
The impact of in utero transfusions on perinatal outcomes in patients with alpha thalassemia major: the UCSF registry
Abstract
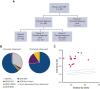
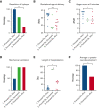
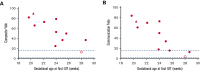
Alpha thalassemia major (ATM) is a hemoglobinopathy that usually results in perinatal demise if in utero transfusions (IUTs) are not performed. We established an international registry (NCT04872179) to evaluate the impact of IUTs on survival to discharge (primary outcome) as well as perinatal and neurodevelopmental secondary outcomes. Forty-nine patients were diagnosed prenatally, 11 were diagnosed postnatally, and all 11 spontaneous survivor genotypes had preserved embryonic zeta-globin levels. We compared 3 groups of patients; group 1, prenatally diagnosed and alive at hospital discharge (n = 14), group 2, prenatally diagnosed and deceased perinatally (n = 5), and group 3, postnatally diagnosed and alive at hospital discharge (n = 11). Group 1 had better outcomes than groups 2 and 3 in terms of the resolution of hydrops, delivery closer to term, shorter hospitalizations, and more frequent average or greater neurodevelopmental outcomes. Earlier IUT initiation was correlated with higher neurodevelopmental (Vineland-3) scores (r = -0.72, P = .02). Preterm delivery after IUT was seen in 3/16 (19%) patients who continued their pregnancy. When we combined our data with those from 2 published series, patients who received ≥2 IUTs had better outcomes than those with 0 to 1 IUT, including resolution of hydrops, delivery at ≥34 weeks gestation, and 5-minute appearance, pulse, grimace, activity, and respiration scores ≥7. Neurodevelopmental assessments were normal in 17/18 of the ≥2 IUT vs 5/13 of the 0 to 1 IUT group (OR 2.74; P = .01). Thus, fetal transfusions enable the survival of patients with ATM and normal neurodevelopment, even in those patients presenting with hydrops. Nondirective prenatal counseling for expectant parents should include the option of IUTs.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: T.C.M. is on the scientific advisory board of Acrigen. The remaining authors declare no competing interests.
Figures


References
-
- Piel FB, Weatherall DJ. The alpha-thalassemias. N Engl J Med. 2014;371(20):1908–1916. - PubMed
-
- Vichinsky E. Complexity of alpha thalassemia: growing health problem with new approaches to screening, diagnosis, and therapy. Ann N Y Acad Sci. 2010;1202:180–187. - PubMed
-
- Farashi S, Harteveld CL. Molecular basis of alpha-thalassemia. Blood Cells Mol Dis. 2018;70:43–53. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

