The Metabolome Weakens RNA Thermodynamic Stability and Strengthens RNA Chemical Stability
- PMID: 36306436
- PMCID: PMC9669196
- DOI: 10.1021/acs.biochem.2c00488
The Metabolome Weakens RNA Thermodynamic Stability and Strengthens RNA Chemical Stability
Erratum in
-
Correction to "The Metabolome Weakens RNA Thermodynamic Stability and Strengthens RNA Chemical Stability".Biochemistry. 2025 Aug 5;64(15):3486. doi: 10.1021/acs.biochem.4c00796. Epub 2025 Jul 15. Biochemistry. 2025. PMID: 40662228 No abstract available.
Abstract
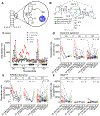
We examined the complex network of interactions among RNA, the metabolome, and divalent Mg2+ under conditions that mimic the Escherichia coli cytoplasm. We determined Mg2+ binding constants for the top 15 E. coli metabolites, comprising 80% of the metabolome by concentration at physiological pH and monovalent ion concentrations. These data were used to inform the development of an artificial cytoplasm that mimics in vivo E. coli conditions, which we term "Eco80". We empirically determined that the mixture of E. coli metabolites in Eco80 approximated single-site binding behavior toward Mg2+ in the biologically relevant free Mg2+ range of ∼0.5 to 3 mM Mg2+, using a Mg2+-sensitive fluorescent dye. Effects of Eco80 conditions on the thermodynamic stability, chemical stability, structure, and catalysis of RNA were examined. We found that Eco80 conditions lead to opposing effects on the thermodynamic and chemical stabilities of RNA. In particular, the thermodynamic stability of RNA helices was weakened by 0.69 ± 0.12 kcal/mol, while the chemical stability was enhanced ∼2-fold, which can be understood using the speciation of Mg2+ between weak and strong Mg2+-metabolite complexes in Eco80. Overall, the use of Eco80 reflects RNA function in vivo and enhances the biological relevance of mechanistic studies of RNA.
Figures

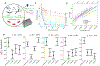


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

