Efficacy and safety of methylphenidate and behavioural parent training for children aged 3-5 years with attention-deficit hyperactivity disorder: a randomised, double-blind, placebo-controlled, and sham behavioural parent training-controlled trial
- PMID: 36306807
- PMCID: PMC9731509
- DOI: 10.1016/S2352-4642(22)00279-6
Efficacy and safety of methylphenidate and behavioural parent training for children aged 3-5 years with attention-deficit hyperactivity disorder: a randomised, double-blind, placebo-controlled, and sham behavioural parent training-controlled trial
Abstract
Background: There is insufficient evidence to support treatment recommendations for preschool children aged 3-5 years with attention-deficit hyperactivity disorder (ADHD). We aimed to investigate the efficacy and safety of methylphenidate and behavioural parent training in reducing the frequency and severity of symptoms and improving global functioning in preschool children with ADHD.
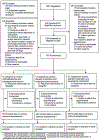
Methods: We did an 8-week, randomised, double-blind, placebo-controlled and sham behavioural parent training-controlled clinical trial (the MAPPA Study) in children aged 3-5 years with moderate-to-severe ADHD. The trial was conducted at the Institute of Psychiatry, Hospital das Clinicas, University of São Paulo Medical School, São Paulo, Brazil. Participants were randomly assigned (1:1:1) to receive immediate-release methylphenidate plus educational intervention (sham behavioural parent training), placebo medication plus behavioural parent training, or placebo medication plus educational intervention. Randomisation was done by an independent research manager by use of a permuted block randomisation procedure. Parents, teachers, study staff, and evaluators remained masked to group allocation. Methylphenidate and placebo were titrated to a maximum dose of 1·25 mg/kg per day administered orally twice daily, and behavioural parent training and the educational intervention were delivered weekly through 90 min sessions with both the child and parent, conducted by two psychologists or learning therapists. The primary outcomes were parents' and teachers' composite scores of the Swanson, Nolan, and Pelham-IV scale (SNAP-IV-P/T), the Clinical Global Impressions Severity (CGI-S) scale, and the Children's Global Assessment Scale (CGAS). This trial is registered with ClinicalTrials.gov, NCT02807870, and is now complete. All participants were invited to participate in an open observational follow-up, which is ongoing.
Findings: Between Aug 21, 2016, and Oct 21, 2019, 153 children were randomly assigned to receive methylphenidate plus the educational intervention (n=51), placebo plus behavioural parent training (n=51), or placebo plus the educational intervention (n=51). Nine (6%) children discontinued treatment. All participants were included in the intention-to-treat analysis. Children in the methylphenidate plus educational intervention group showed greater reductions in the SNAP-IV-P/T (endpoint mean difference -3·93 [95% CI -7·14 to -0·73], p=0·049; effect size -0·55 [95% CI -0·99 to -0·10]) and CGI-S scores (endpoint mean difference -0·49 [-0·82 to -0·17], p=0·0088; effect size -0·70 [-1·16 to -0·24]) and a greater increase in CGAS scores (endpoint mean difference 5·25 [95% CI 2·09 to 8·40], p=0·0036; effect size 0·80 [95% CI 0·32 to 1·28]) than children in the placebo plus educational intervention group. Children in the placebo plus behavioural parent training group did not have significantly different SNAP-IV-P/T scores (endpoint mean difference -3·18 [95% CI -6·38 to 0·02], p=0·077; effect size -0·44 [95% CI -0·89 to 0·003]) or CGI-S scores (endpoint mean difference -0·35 [-0·68 to -0·03], p=0·052; effect size -0·50 [-0·96 to -0·04]) compared to children in the placebo plus educational intervention group, but they had a greater increase in CGAS scores compared to the placebo plus educational intervention group (endpoint mean difference 3·69 [0·53 to 6·85], p=0·033; effect size 0·56 [0·08 to 1·04]). Children in the methylphenidate plus educational intervention versus placebo plus behavioural parent training group did not have statistically or clinically significant differences in primary outcomes. Children in the methylphenidate plus educational intervention group had more mild adverse events than the other two groups, and there were no between-group differences for moderate or severe adverse events.
Interpretation: Methylphenidate was effective in reducing ADHD symptoms and improving functionality, and behavioural parent training was effective in improving functionality for preschool children with ADHD after 8 weeks of treatment.
Funding: São Paulo Research Foundation and Brazilian National Council for Scientific and Technological Development.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests GVP has, in the past 3 years, been a consultant, member of advisory board, or speaker, or a combination of the above, for Takeda, Medice, Aché, Novo Nordisk, and Abbott; and has received royalties from Editora Manole. LAR has received grant or research support from, served as a consultant to, and served on the speakers' bureau of Aché, Bial, Medice, Novartis/ Sandoz, Pfizer/Upjohn, and Shire/Takeda in the past 3 years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by LAR have received unrestricted educational and research support from the following pharmaceutical companies in the past 3 years: Novartis/Sandoz and Shire/Takeda. LAR has received authorship royalties from Oxford University Press and ArtMed. All other authors declare no competing interests.
Figures
Comment in
-
Treatment of ADHD in preschool children.Lancet Child Adolesc Health. 2022 Dec;6(12):830-831. doi: 10.1016/S2352-4642(22)00312-1. Epub 2022 Oct 26. Lancet Child Adolesc Health. 2022. PMID: 36306806 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical