Liver Iron Loading in Alcohol-Associated Liver Disease
- PMID: 36306827
- PMCID: PMC12178325
- DOI: 10.1016/j.ajpath.2022.08.010
Liver Iron Loading in Alcohol-Associated Liver Disease
Abstract
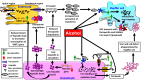
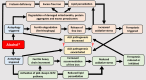
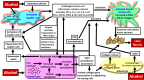
Alcohol-associated liver disease (ALD) is a common chronic liver disease with increasing incidence worldwide. Alcoholic liver steatosis/steatohepatitis can progress to liver fibrosis/cirrhosis, which can cause predisposition to hepatocellular carcinoma. ALD diagnosis and management are confounded by several challenges. Iron loading is a feature of ALD which can exacerbate alcohol-induced liver injury and promote ALD pathologic progression. Knowledge of the mechanisms that mediate liver iron loading can help identify cellular/molecular targets and thereby aid in designing adjunct diagnostic, prognostic, and therapeutic approaches for ALD. Herein, the cellular mechanisms underlying alcohol-induced liver iron loading are reviewed and how excess iron in patients with ALD can promote liver fibrosis and aggravate disease pathology is discussed. Alcohol-induced increase in hepatic transferrin receptor-1 expression and up-regulation of high iron protein in Kupffer cells (proposed) facilitate iron deposition and retention in the liver. Iron is loaded in both parenchymal and nonparenchymal liver cells. Iron-loaded liver can promote ferroptosis and thereby contribute to ALD pathology. Iron and alcohol can independently elevate oxidative stress. Therefore, a combination of excess iron and alcohol amplifies oxidative stress and accelerates liver injury. Excess iron-stimulated hepatocytes directly or indirectly (through Kupffer cell activation) activate the hepatic stellate cells via secretion of proinflammatory and profibrotic factors. Persistently activated hepatic stellate cells promote liver fibrosis, and thereby facilitate ALD progression.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
The Cellular, Molecular, and Pathologic Consequences of Stress on the Liver.Am J Pathol. 2023 Oct;193(10):1353-1354. doi: 10.1016/j.ajpath.2023.07.003. Epub 2023 Aug 4. Am J Pathol. 2023. PMID: 37544504 Free PMC article. No abstract available.
References
-
- Manthey J., Shield K.D., Rylett M., Hasan O.S.M., Probst C., Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393:2493–2502. - PubMed
-
- Ganne-Carrié N., Christidis C., Chastang C., Ziol M., Chapel F., Imbert-Bismut F., Trinchet J.C., Guettier C., Beaugrand M. Liver iron is predictive of death in alcoholic cirrhosis: a multivariate study of 229 consecutive patients with alcoholic and/or hepatitis C virus cirrhosis: a prospective follow up study. Gut. 2000;46:277–282. - PMC - PubMed
-
- Eng S.C., Taylor S.L., Reyes V., Raaka S., Berger J., Kowdley K.V. Hepatic iron overload in alcoholic end-stage liver disease is associated with iron deposition in other organs in the absence of HFE-1 hemochromatosis. Liver Int. 2005;25:513–517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

