Motion-corrected 4D-Flow MRI for neurovascular applications
- PMID: 36307060
- PMCID: PMC9801539
- DOI: 10.1016/j.neuroimage.2022.119711
Motion-corrected 4D-Flow MRI for neurovascular applications
Abstract
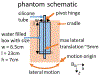
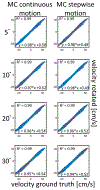
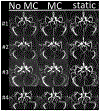
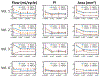
Neurovascular 4D-Flow MRI has emerged as a powerful tool for comprehensive cerebrovascular hemodynamic characterization. Clinical studies in at risk populations such as aging adults indicate hemodynamic markers can be confounded by motion-induced bias. This study develops and characterizes a high fidelity 3D self-navigation approach for retrospective rigid motion correction of neurovascular 4D-Flow data. A 3D radial trajectory with pseudorandom ordering was combined with a multi-resolution low rank regularization approach to enable high spatiotemporal resolution self-navigators from extremely undersampled data. Phantom and volunteer experiments were performed at 3.0T to evaluate the ability to correct for different amounts of induced motions. In addition, the approach was applied to clinical-research exams from ongoing aging studies to characterize performance in the clinical setting. Simulations, phantom and volunteer experiments with motion correction produced images with increased vessel conspicuity, reduced image blurring, and decreased variability in quantitative measures. Clinical exams revealed significant changes in hemodynamic parameters including blood flow rates, flow pulsatility index, and lumen areas after motion correction in probed cerebral arteries (Flow: P<0.001 Lt ICA, P=0.002 Rt ICA, P=0.004 Lt MCA, P=0.004 Rt MCA; Area: P<0.001 Lt ICA, P<0.001 Rt ICA, P=0.004 Lt MCA, P=0.004 Rt MCA; flow pulsatility index: P=0.042 Rt ICA, P=0.002 Lt MCA). Motion induced bias can lead to significant overestimation of hemodynamic markers in cerebral arteries. The proposed method reduces measurement bias from rigid motion in neurovascular 4D-Flow MRI in challenging populations such as aging adults.
Keywords: 4D Flow MRI; Angiography; Bias; Head motion; Motion correction; Quality control.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest S.C. Johnson serves on an advisory board for Roche Diagnostics for which he receives an honorarium and is principal investigator of an equipment grant from Roche. He receives research funding from Cerveau Technologies
Figures











Similar articles
-
Accelerated dual-venc 4D flow MRI for neurovascular applications.J Magn Reson Imaging. 2017 Jul;46(1):102-114. doi: 10.1002/jmri.25595. Epub 2017 Feb 2. J Magn Reson Imaging. 2017. PMID: 28152256 Free PMC article.
-
Intracranial artery velocity measurement using 4D PC MRI at 3 T: comparison with transcranial ultrasound techniques and 2D PC MRI.Neuroradiology. 2013 Mar;55(4):389-98. doi: 10.1007/s00234-012-1103-z. Epub 2012 Nov 10. Neuroradiology. 2013. PMID: 23143179
-
Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries.J Magn Reson Imaging. 2015 Nov;42(5):1458-64. doi: 10.1002/jmri.24900. Epub 2015 Apr 2. J Magn Reson Imaging. 2015. PMID: 25847621 Free PMC article.
-
From 2D to 4D Phase-Contrast MRI in the Neurovascular System: Will It Be a Quantum Jump or a Fancy Decoration?J Magn Reson Imaging. 2022 Feb;55(2):347-372. doi: 10.1002/jmri.27430. Epub 2020 Nov 24. J Magn Reson Imaging. 2022. PMID: 33236488 Review.
-
Advances in 4D medical imaging and 4D radiation therapy.Technol Cancer Res Treat. 2008 Feb;7(1):67-81. doi: 10.1177/153303460800700109. Technol Cancer Res Treat. 2008. PMID: 18198927 Review.
Cited by
-
Four-dimensional flow MRI for quantitative assessment of cerebrospinal fluid dynamics: Status and opportunities.NMR Biomed. 2024 Jul;37(7):e5082. doi: 10.1002/nbm.5082. Epub 2023 Dec 20. NMR Biomed. 2024. PMID: 38124351 Free PMC article. Review.
-
Respiratory influence on cerebral blood flow and blood volume - A 4D flow MRI study.J Cereb Blood Flow Metab. 2025 Aug;45(8):1531-1542. doi: 10.1177/0271678X251316395. Epub 2025 Jan 30. J Cereb Blood Flow Metab. 2025. PMID: 39883800 Free PMC article.
-
Cerebral artery and brain pathology correlates of antemortem cerebral artery 4D flow MRI.Imaging Neurosci (Camb). 2024;2:imag-2-00322. doi: 10.1162/imag_a_00322. Epub 2024 Oct 25. Imaging Neurosci (Camb). 2024. PMID: 40092424 Free PMC article.
-
Unraveling diurnal and technical variability in cerebral hemodynamics from neurovascular 4D-Flow MRI.J Cereb Blood Flow Metab. 2024 Aug;44(8):1362-1375. doi: 10.1177/0271678X241232190. Epub 2024 Feb 10. J Cereb Blood Flow Metab. 2024. PMID: 38340787 Free PMC article.
-
Highly accelerated 4D flow MRI with respiratory compensation and cardiac view sharing: a cross-sectional study of flow in the great vessels of pediatric congenital heart disease.Pediatr Radiol. 2025 May;55(6):1223-1234. doi: 10.1007/s00247-025-06226-1. Epub 2025 Apr 5. Pediatr Radiol. 2025. PMID: 40186653
References
-
- Aristova M, Vali A, Ansari SA, Shaibani A, Alden TD, Hurley MC, Jahromi BS, Potts MB, Markl M, Schnell S, 2019. Standardized Evaluation of Cerebral Arteriovenous Malformations Using Flow Distribution Network Graphs and Dual-venc 4D Flow MRI. J Magn Reson Imaging 50, 1718–1730. 10.1002/jmri.26784 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

