Risk of Multiple Sclerosis Among Users of Antitumor Necrosis Factor α in 4 Canadian Provinces: A Population-Based Study
- PMID: 36307225
- PMCID: PMC9946189
- DOI: 10.1212/WNL.0000000000201472
Risk of Multiple Sclerosis Among Users of Antitumor Necrosis Factor α in 4 Canadian Provinces: A Population-Based Study
Abstract
Background and objectives: Antitumor necrosis factor α (TNFα) agents are a class of biologic drugs used for the treatment of several immune-mediated conditions. An increased risk of multiple sclerosis (MS) with their use has been suggested, but studies have been limited. Relevant population-based epidemiologic data linking anti-TNFα to MS are scarce. The objective was to compare the risk of MS in anti-TNFα users with nonusers among patients with rheumatic disease (RD) or inflammatory bowel disease (IBD).
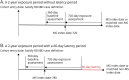
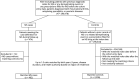
Methods: A nested case-control study was conducted. Population-based health care-linked databases from 4 Canadian provinces were used. All patients with RD or IBD residing within a participating province between January 2000 and March 2018 were identified by validated case definitions. Any anti-TNFα dispensation in the 2 years before the index date (MS onset) was identified. Incident onset MS cases were ascertained using a validated algorithm. Up to 5 controls were matched to each MS case based on birth year ±3 years, disease duration, and health authority (based on region of residence). Conditional logistic regressions were used to calculate the incidence rate ratio (IRR) after adjusting for potential confounders. A meta-analysis was conducted to provide pooled estimates across provinces using random-effects models.
Results: Among 296,918 patients with RD patients, 462 MS cases (80.1% female, mean [SD] age, 47.4 [14.6] years) were matched with 2,296 controls (59.5% female, mean [SD] age, 47.4 [14.5] years). Exposure to anti-TNFα occurred in 18 MS cases and 42 controls. After adjusting for matching variables, sex, and the Charlson Comorbidity Index, the pooled IRR was 2.05 (95% CI, 1.13-3.72). Among 84,458 patients with IBD, 190 MS cases (69.5% female, mean [SD] age, 44.3 [12.3] years) were matched with 943 controls (54.1% female, mean [SD] age, 44.2 [12.2] years). Exposure to anti-TNFα occurred in 23 MS cases and 98 controls. The pooled adjusted IRR was 1.35 (95% CI, 0.70-2.59).
Discussion: The use of anti-TNFα was associated with an increased risk of MS compared with nonusers, especially among patients with RD. These findings could help clinicians and patients with RD to make more informed treatment decisions. Further studies are needed to validate these results for patients with IBD.
© 2022 American Academy of Neurology.
Figures


Comment in
-
Tumor Necrosis Factor α Blockers and the Risk of Multiple Sclerosis.Neurology. 2023 Feb 7;100(6):267-268. doi: 10.1212/WNL.0000000000201629. Epub 2022 Oct 28. Neurology. 2023. PMID: 36307218 No abstract available.
References
-
- Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled. Arthritis Rheum. 2004;50(5):1400-1411. doi: 10.1002/art.20217 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
