The immune synapses reveal aberrant functions of CD8 T cells during chronic HIV infection
- PMID: 36307445
- PMCID: PMC9616955
- DOI: 10.1038/s41467-022-34157-0
The immune synapses reveal aberrant functions of CD8 T cells during chronic HIV infection
Abstract
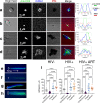
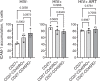
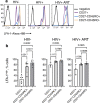
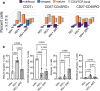
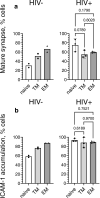
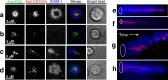
Chronic HIV infection causes persistent low-grade inflammation that induces premature aging of the immune system including senescence of memory and effector CD8 T cells. To uncover the reasons of gradually diminished potency of CD8 T cells from people living with HIV, here we expose the T cells to planar lipid bilayers containing ligands for T-cell receptor and a T-cell integrins and analyze the cellular morphology, dynamics of synaptic interface formation and patterns of the cellular degranulation. We find a large fraction of phenotypically naive T cells from chronically infected people are capable to form mature synapse with focused degranulation, a signature of a differentiated T cells. Further, differentiation of aberrant naive T cells may lead to the development of anomalous effector T cells undermining their capacity to control HIV and other pathogens that could be contained otherwise.
© 2022. The Author(s).
Conflict of interest statement
M.R.B. is a consultant for Interius BioTherapeutics. No other conflicts are reported by the authors.
Figures

Similar articles
-
Partial recovery of senescence and differentiation disturbances in CD8+ T cell effector-memory cells in HIV-1 infection after initiation of anti-retroviral treatment.Clin Exp Immunol. 2016 Nov;186(2):227-238. doi: 10.1111/cei.12837. Epub 2016 Aug 23. Clin Exp Immunol. 2016. PMID: 27377704 Free PMC article.
-
Impact of Aging, Cytomegalovirus Infection, and Long-Term Treatment for Human Immunodeficiency Virus on CD8+ T-Cell Subsets.Front Immunol. 2018 Mar 21;9:572. doi: 10.3389/fimmu.2018.00572. eCollection 2018. Front Immunol. 2018. PMID: 29619031 Free PMC article.
-
HIV replication leads to skewed maturation of CD8-positive T-cell responses in infected children.New Microbiol. 2010 Oct;33(4):303-9. New Microbiol. 2010. PMID: 21213588
-
Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection.PLoS Biol. 2004 Feb;2(2):E20. doi: 10.1371/journal.pbio.0020020. Epub 2004 Feb 17. PLoS Biol. 2004. PMID: 14966528 Free PMC article. Review.
-
Accelerated immune senescence and HIV-1 infection.Exp Gerontol. 2007 May;42(5):432-7. doi: 10.1016/j.exger.2006.12.003. Epub 2007 Jan 8. Exp Gerontol. 2007. PMID: 17307327 Review.
Cited by
-
A new perspective on HIV: effects of HIV on brain-heart axis.Front Cardiovasc Med. 2023 Aug 4;10:1226782. doi: 10.3389/fcvm.2023.1226782. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37600062 Free PMC article. Review.
-
Classification of T lymphocyte motility behaviors using a machine learning approach.PLoS Comput Biol. 2023 Sep 11;19(9):e1011449. doi: 10.1371/journal.pcbi.1011449. eCollection 2023 Sep. PLoS Comput Biol. 2023. PMID: 37695797 Free PMC article.
-
The single-cell immune landscape of HIV-associated aggressive B-cell lymphoma.J Natl Cancer Cent. 2025 Feb 12;5(2):221-235. doi: 10.1016/j.jncc.2025.02.001. eCollection 2025 Apr. J Natl Cancer Cent. 2025. PMID: 40265092 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

