Nebulized fusion inhibitory peptide protects cynomolgus macaques from measles virus infection
- PMID: 36307480
- PMCID: PMC9616412
- DOI: 10.1038/s41467-022-33832-6
Nebulized fusion inhibitory peptide protects cynomolgus macaques from measles virus infection
Abstract
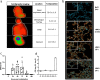
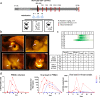
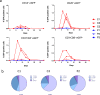
Measles is the most contagious airborne viral infection and the leading cause of child death among vaccine-preventable diseases. We show here that aerosolized lipopeptide fusion inhibitor, derived from heptad-repeat regions of the measles virus (MeV) fusion protein, blocks respiratory MeV infection in a non-human primate model, the cynomolgus macaque. We use a custom-designed mesh nebulizer to ensure efficient aerosol delivery of peptide to the respiratory tract and demonstrate the absence of adverse effects and lung pathology in macaques. The nebulized peptide efficiently prevents MeV infection, resulting in the complete absence of MeV RNA, MeV-infected cells, and MeV-specific humoral responses in treated animals. This strategy provides an additional means to fight against respiratory infection in non-vaccinated people, that can be readily translated to human trials. It presents a proof-of-concept for the aerosol delivery of fusion inhibitory peptides to protect against measles and other airborne viruses, including SARS-CoV-2, in case of high-risk exposure.
© 2022. The Author(s).
Conflict of interest statement
S. Le Guellec is employed by DTF Medical (Saint Etienne, France) and L. Vecellio was employed by DTF Medical from 2001 to 2018 and by Nemera (La Verpilliere, France) from 2018 to 2020. The remaining authors declare no competing interests.
Figures




Update of
-
Nebulized fusion inhibitory peptide protects cynomolgus macaques from measles virus infection.Res Sq [Preprint]. 2022 Jun 1:rs.3.rs-1700877. doi: 10.21203/rs.3.rs-1700877/v1. Res Sq. 2022. Update in: Nat Commun. 2022 Oct 28;13(1):6439. doi: 10.1038/s41467-022-33832-6. PMID: 35677066 Free PMC article. Updated. Preprint.
References
-
- Anderson RM, May RM. Directly transmitted infections diseases: control by vaccination. Science. 1982;215:1053–1060. - PubMed
-
- Durrheim DN, et al. A dangerous measles future looms beyond the COVID-19 pandemic. Nat. Med. 2021;27:360–361. - PubMed
-
- Roberts L. Why measles deaths are surging—and coronavirus could make it worse. Nature. 2020;580:446–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

