The ex vivo human translaminar autonomous system to study spaceflight associated neuro-ocular syndrome pathogenesis
- PMID: 36307487
- PMCID: PMC9616431
- DOI: 10.1038/s41526-022-00232-5
The ex vivo human translaminar autonomous system to study spaceflight associated neuro-ocular syndrome pathogenesis
Abstract
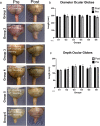
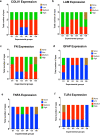
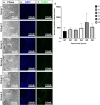
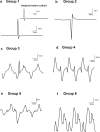
Spaceflight-Associated Neuro-ocular Syndrome (SANS) is a significant unexplained adverse reaction to long-duration spaceflight. We employ an ex vivo translaminar autonomous system (TAS) to recreate a human ocular ground-based spaceflight analogue model to study SANS pathogenesis. To recapitulate the human SANS conditions, human ocular posterior segments are cultured in the TAS model for 14 days. Translaminar pressure differentials are generated by simulating various flow rates within intracranial pressure (ICP) and intraocular (IOP) chambers to maintain hydrostatic pressures of ICP: IOP (12:16, 15:16, 12:21, 21:16 mmHg). In addition, optic nerves are mechanically kinked by 6- and 10-degree tilt inserts for the ICP: IOP;15:16 mmHg pressure paradigm. The TAS model successfully maintains various pressure differentials for all experimental groups over 14 days. Post culture, we determine inflammatory and extracellular component expression changes within posterior segments. To further characterize the SANS pathogenesis, axonal transport capacity, optic nerve degeneration and retinal functional are measured. Identifiable pathogenic alterations are observed in posterior segments by morphologic, apoptotic, and inflammatory changes including transport and functional deficits under various simulated SANS conditions. Here we report our TAS model provides a unique preclinical application system to mimic SANS pathology and a viable therapeutic testing device for countermeasures.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing non-financial interests but the following competing financial interests for the patent application of the Translaminar Autonomous System is under the patent application-U.S. Patent Application No. 16/395,610.
Figures






References
-
- Kramer LA, Sargsyan AE, Hasan KM, Polk JD, Hamilton DR. Orbital and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology. 2012;263:819–827. - PubMed
-
- Mader TH, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118:2058–2069. - PubMed
-
- Michael AP, Marshall-Bowman K. Spaceflight-induced intracranial hypertension. Aerosp. Med. Hum. Perform. 2015;86:557–562. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

