Dysregulated transforming growth factor-beta mediates early bone marrow dysfunction in diabetes
- PMID: 36307522
- PMCID: PMC9616825
- DOI: 10.1038/s42003-022-04112-2
Dysregulated transforming growth factor-beta mediates early bone marrow dysfunction in diabetes
Abstract
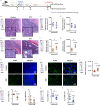
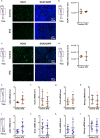
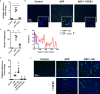
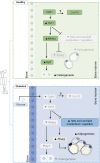
Diabetes affects select organs such as the eyes, kidney, heart, and brain. Our recent studies show that diabetes also enhances adipogenesis in the bone marrow and reduces the number of marrow-resident vascular regenerative stem cells. In the current study, we have performed a detailed spatio-temporal examination to identify the early changes that are induced by diabetes in the bone marrow. Here we show that short-term diabetes causes structural and molecular changes in the marrow, including enhanced adipogenesis in tibiae of mice, prior to stem cell depletion. This enhanced adipogenesis was associated with suppressed transforming growth factor-beta (TGFB) signaling. Using human bone marrow-derived mesenchymal progenitor cells, we show that TGFB pathway suppresses adipogenic differentiation through TGFB-activated kinase 1 (TAK1). These findings may inform the development of novel therapeutic targets for patients with diabetes to restore regenerative stem cell function.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/beta-catenin signaling pathways.J Biol Chem. 2008 Apr 4;283(14):9136-45. doi: 10.1074/jbc.M708968200. Epub 2008 Jan 18. J Biol Chem. 2008. PMID: 18203713 Free PMC article.
-
Knockdown of CDC20 promotes adipogenesis of bone marrow-derived stem cells by modulating β-catenin.Stem Cell Res Ther. 2022 Sep 2;13(1):443. doi: 10.1186/s13287-022-03062-0. Stem Cell Res Ther. 2022. PMID: 36056439 Free PMC article.
-
TGF-β Signaling Plays an Essential Role in the Lineage Specification of Mesenchymal Stem/Progenitor Cells in Fetal Bone Marrow.Stem Cell Reports. 2019 Jul 9;13(1):48-60. doi: 10.1016/j.stemcr.2019.05.017. Epub 2019 Jun 13. Stem Cell Reports. 2019. PMID: 31204302 Free PMC article.
-
Roles of MicroRNAs in Osteogenesis or Adipogenesis Differentiation of Bone Marrow Stromal Progenitor Cells.Int J Mol Sci. 2021 Jul 5;22(13):7210. doi: 10.3390/ijms22137210. Int J Mol Sci. 2021. PMID: 34281266 Free PMC article. Review.
-
The transforming growth factor-beta/bone morphogenetic protein signalling pathway in adipogenesis.Int J Biochem Cell Biol. 2012 Mar;44(3):475-9. doi: 10.1016/j.biocel.2011.12.014. Epub 2011 Dec 31. Int J Biochem Cell Biol. 2012. PMID: 22226816 Review.
Cited by
-
Effects of Combustible Cigarettes and Heated Tobacco Products on Systemic Inflammatory Response in Patients with Chronic Inflammatory Diseases.Diseases. 2024 Jul 5;12(7):144. doi: 10.3390/diseases12070144. Diseases. 2024. PMID: 39057115 Free PMC article.
-
The Description and Prediction of Incidence, Prevalence, Mortality, Disability-Adjusted Life Years Cases, and Corresponding Age-Standardized Rates for Global Diabetes.J Epidemiol Glob Health. 2023 Sep;13(3):566-576. doi: 10.1007/s44197-023-00138-9. Epub 2023 Jul 3. J Epidemiol Glob Health. 2023. PMID: 37400673 Free PMC article.
-
Considering the Cellular Landscape in Marrow Stimulation Techniques for Cartilage Repair.Cells Tissues Organs. 2024;213(6):523-537. doi: 10.1159/000538530. Epub 2024 Apr 10. Cells Tissues Organs. 2024. PMID: 38599194 Free PMC article. Review.
-
Differentially Expressed Genes Regulating Glutathione Metabolism, Protein-Folding, and Unfolded Protein Response in Pancreatic β-Cells in Type 2 Diabetes Mellitus.Int J Mol Sci. 2023 Jul 27;24(15):12059. doi: 10.3390/ijms241512059. Int J Mol Sci. 2023. PMID: 37569434 Free PMC article.
References
-
- Ogurtsova K, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin. Pr. 2017;128:40–50. - PubMed
-
- Santopaolo M, Sambataro M, Spinetti G, Madeddu P. Bone marrow as a target and accomplice of vascular complications in diabetes. Diabetes Metab. Res Rev. 2020;36(Suppl 1):e3240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

