SAM-X: sorting algorithm for musculoskeletal x-ray radiography
- PMID: 36307553
- PMCID: PMC9935683
- DOI: 10.1007/s00330-022-09184-6
SAM-X: sorting algorithm for musculoskeletal x-ray radiography
Abstract
Objective: To develop a two-phased deep learning sorting algorithm for post-X-ray image acquisition in order to facilitate large musculoskeletal image datasets according to their anatomical entity.
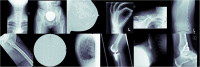
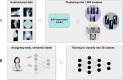
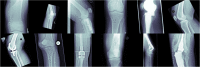
Methods: In total, 42,608 unstructured and pseudonymized radiographs were retrieved from the PACS of a musculoskeletal tumor center. In phase 1, imaging data were sorted into 1000 clusters by a self-supervised model. A human-in-the-loop radiologist assigned weak, semantic labels to all clusters and clusters with the same label were merged. Three hundred thirty-two non-musculoskeletal clusters were discarded. In phase 2, the initial model was modified by "injecting" the identified labels into the self-supervised model to train a classifier. To provide statistical significance, data split and cross-validation were applied. The hold-out test set consisted of 50% external data. To gain insight into the model's predictions, Grad-CAMs were calculated.
Results: The self-supervised clustering resulted in a high normalized mutual information of 0.930. The expert radiologist identified 28 musculoskeletal clusters. The modified model achieved a classification accuracy of 96.2% and 96.6% for validation and hold-out test data for predicting the top class, respectively. When considering the top two predicted class labels, an accuracy of 99.7% and 99.6% was accomplished. Grad-CAMs as well as final cluster results underlined the robustness of the proposed method by showing that it focused on similar image regions a human would have considered for categorizing images.
Conclusion: For efficient dataset building, we propose an accurate deep learning sorting algorithm for classifying radiographs according to their anatomical entity in the assessment of musculoskeletal diseases.
Key points: • Classification of large radiograph datasets according to their anatomical entity. • Paramount importance of structuring vast amounts of retrospective data for modern deep learning applications. • Optimization of the radiological workflow and increase in efficiency as well as decrease of time-consuming tasks for radiologists through deep learning.
Keywords: Artificial intelligence; Deep learning; Musculoskeletal diseases; Workflow; X-ray.
© 2022. The Author(s).
Conflict of interest statement
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Figures

References
-
- Organization, W.H . The burden of musculoskeletal conditions at the start of the new millennium: report of a WHO scientific group. 2003. - PubMed
-
- Board, W.C.o.T.E., Soft Tissue and Bone Tumours (2020) International agency for research on cancer
-
- Liu X, Faes L, Kale AU et al (2019) A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health 1(6):e271–e297 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

