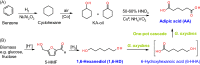
A facile process for adipic acid production in high yield by oxidation of 1,6-hexanediol using the resting cells of Gluconobacter oxydans
- PMID: 36307807
- PMCID: PMC9617331
- DOI: 10.1186/s12934-022-01947-6
A facile process for adipic acid production in high yield by oxidation of 1,6-hexanediol using the resting cells of Gluconobacter oxydans
Abstract
Background: Adipic acid (AA) is one of the most important industrial chemicals used mainly for the production of Nylon 6,6 but also for making polyurethanes, plasticizers, and unsaturated polyester resins, and more recently as a component in the biodegradable polyester poly(butylene adipate terephthalate) (PBAT). The main route for AA production utilizes benzene as feedstock and generates copious amounts of the greenhouse gas NO2. Hence, alternative clean production routes for AA from renewable bio-based feedstock are drawing increasing attention. We have earlier reported the potential of Gluconobacter oxydans cells to oxidize 1,6-hexanediol, a potentially biobased diol to AA.
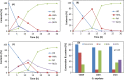
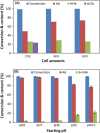
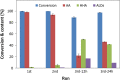
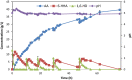
Results: The present report involves a study on the effect of different parameters on the microbial transformation of 1,6-hexanediol to adipic acid, and subsequently testing the process on a larger lab scale for achieving maximal conversion and yield. Comparison of three wild-type strains of G. oxydans DSM50049, DSM2003, and DSM2343 for the whole-cell biotransformation of 10 g/L 1,6-hexanediol to adipic acid in batch mode at pH 7 and 30 °C led to the selection of G. oxydans DSM50049, which showed 100% conversion of the substrate with over 99% yield of adipic acid in 30 h. An increase in the concentrations of the substrate decreased the degree of conversion, while the product up to 25 g/L in batch and 40 g/L in fed-batch showed no inhibition on the conversion. Moreover, controlling the pH of the reaction at 5-5.5 was required for the cascade oxidation reactions to work. Cell recycling for the biotransformation resulted in a significant decrease in activity during the third cycle. Meanwhile, the fed-batch mode of transformation by intermittent addition of 1,6-hexanediol (30 g in total) in 1 L scale resulted in complete conversion with over 99% yield of adipic acid (approximately 37 g/L). The product was recovered in a pure form using downstream steps without the use of any solvent.
Conclusion: A facile, efficient microbial process for oxidation of 1,6-hexanediol to adipic acid, having potential for scale up was demonstrated. The entire process is performed in aqueous medium at ambient temperatures with minimal greenhouse gas emissions. The enzymes involved in catalyzing the oxidation steps are currently being identified.
Keywords: 1,6-hexanediol; Adipic acid; Gluconobacter oxidation; Polymer building block; Whole cell oxidation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Gluconobacter oxydans DSM 50049 - an efficient biocatalyst for oxidation of 5-formyl-2-furancarboxylic acid (FFCA) to 2,5-furandicarboxylic acid (FDCA).Microb Cell Fact. 2025 Mar 19;24(1):68. doi: 10.1186/s12934-025-02689-x. Microb Cell Fact. 2025. PMID: 40108655 Free PMC article.
-
Overexpression of membrane-bound gluconate-2-dehydrogenase to enhance the production of 2-keto-D-gluconic acid by Gluconobacter oxydans.Microb Cell Fact. 2016 Jul 9;15(1):121. doi: 10.1186/s12934-016-0521-8. Microb Cell Fact. 2016. PMID: 27392695 Free PMC article.
-
L-Erythrulose production with a multideletion strain of Gluconobacter oxydans.Appl Microbiol Biotechnol. 2019 Jun;103(11):4393-4404. doi: 10.1007/s00253-019-09824-w. Epub 2019 Apr 18. Appl Microbiol Biotechnol. 2019. PMID: 31001743
-
Biobased adipic acid - The challenge of developing the production host.Biotechnol Adv. 2018 Dec;36(8):2248-2263. doi: 10.1016/j.biotechadv.2018.10.012. Epub 2018 Oct 30. Biotechnol Adv. 2018. PMID: 30389426 Review.
-
Asymmetric oxidation by Gluconobacter oxydans.Appl Microbiol Biotechnol. 2006 Mar;70(2):135-9. doi: 10.1007/s00253-005-0307-0. Epub 2006 Jan 24. Appl Microbiol Biotechnol. 2006. PMID: 16432743 Review.
Cited by
-
From Glucose to Green Chemistry: Breakthrough in Microbial Production of Tartaric Semialdehyde.Microb Biotechnol. 2025 Apr;18(4):e70149. doi: 10.1111/1751-7915.70149. Microb Biotechnol. 2025. PMID: 40266016 Free PMC article.
-
Gluconobacter oxydans DSM 50049 - an efficient biocatalyst for oxidation of 5-formyl-2-furancarboxylic acid (FFCA) to 2,5-furandicarboxylic acid (FDCA).Microb Cell Fact. 2025 Mar 19;24(1):68. doi: 10.1186/s12934-025-02689-x. Microb Cell Fact. 2025. PMID: 40108655 Free PMC article.
-
Optimized enantioselective (S)-2-hydroxypropiophenone synthesis by free- and encapsulated-resting cells of Pseudomonas putida.Microb Cell Fact. 2023 May 3;22(1):89. doi: 10.1186/s12934-023-02073-7. Microb Cell Fact. 2023. PMID: 37131175 Free PMC article.
References
-
- Hatti-Kaul R, Nilsson LJ, Zhang B, Rehnberg N, Lundmark S. Designing biobased recyclable polymers for plastics. Trends Biotechnol. 2020;38(1):50–67. - PubMed
-
- Zhu Y, Romain C, Williams CK. Sustainable polymers from renewable resources. Nature. 2016;540(7633):354–362. - PubMed
-
- Iwata T. Biodegradable and bio-based polymers: future prospects of eco-friendly plastics. Angew Chem Int Ed. 2015;54(11):3210–3215. - PubMed
-
- Mohanty AK, Vivekanandhan S, Pin J-M, Misra M. Composites from renewable and sustainable resources: challenges and innovations. Science. 2018;362(6414):536–542. - PubMed
-
- Straathof AJ, Wahl SA, Benjamin KR, Takors R, Wierckx N, Noorman HJ. Grand research challenges for sustainable industrial biotechnology. Trends Biotechnol. 2019;37(10):1042–1050. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

