The tapetal tissue is essential for the maintenance of redox homeostasis during microgametogenesis in tomato
- PMID: 36307971
- PMCID: PMC10100220
- DOI: 10.1111/tpj.16014
The tapetal tissue is essential for the maintenance of redox homeostasis during microgametogenesis in tomato
Abstract
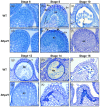
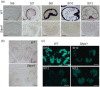
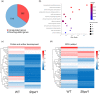
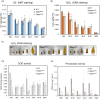
The tapetum is a specialized layer of cells within the anther, adjacent to the sporogenous tissue. During its short life, it provides nutrients, molecules and materials to the pollen mother cells and microsporocytes, being essential during callose degradation and pollen wall formation. The interaction between the tapetum and sporogenous cells in Solanum lycopersicum (tomato) plants, despite its importance for breeding purposes, is poorly understood. To investigate this process, gene editing was used to generate loss-of-function mutants that showed the complete and specific absence of tapetal cells. These plants were obtained targeting the previously uncharacterized Solyc03g097530 (SlTPD1) gene, essential for tapetum specification in tomato plants. In the absence of tapetum, sporogenous cells developed and callose deposition was observed. However, sporocytes failed to undergo the process of meiosis and finally degenerated, leading to male sterility. Transcriptomic analysis conducted in mutant anthers lacking tapetum revealed the downregulation of a set of genes related to redox homeostasis. Indeed, mutant anthers showed a reduction in the accumulation of reactive oxygen species (ROS) at early stages and altered activity of ROS-scavenging enzymes. The results obtained highlight the importance of the tapetal tissue in maintaining redox homeostasis during male gametogenesis in tomato plants.
Keywords: Solanum lycopersicum; TPD1; ROS; anther; male sterility; pollen; tapetum.
© 2022 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest associated with this work.
Figures




References
-
- Aguirre, P.J. & Smith, A.G. (1993) Molecular characterization of a gene encoding a cysteine‐rich protein preferentially expressed in anthers of Lycopersicon esculentum. Plant Molecular Biology, 23, 477–487. - PubMed
-
- Bao, H. , Ding, Y. , Yang, F. , Zhang, J. , Xie, J. , Zhao, C. et al. (2022) Gene silencing, knockout and over‐expression of a transcription factor ABORTED MICROSPORES (SlAMS) strongly affects pollen viability in tomato (Solanum lycopersicum). BMC Genomics, 23, 1–14. 10.1186/s12864-022-08549-x - DOI - PMC - PubMed

