Delivery of topical gentamicin cream via platform wound device to reduce wound infection-A prospective, controlled, randomised, clinical study
- PMID: 36307989
- PMCID: PMC10088835
- DOI: 10.1111/iwj.13998
Delivery of topical gentamicin cream via platform wound device to reduce wound infection-A prospective, controlled, randomised, clinical study
Abstract
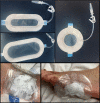
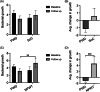
The platform wound device (PWD) is a wound coverage system that is designed to decrease wound infection rates by allowing for direct delivery of topical antibiotics and antimicrobials while creating a sealed, protective barrier around the area of injury. This study evaluated the safety and efficacy of the PWD as a protective dressing and a delivery system for topical antibiotics compared to the current standard of care (SoC). This was a multi-center, prospective, randomised, controlled clinical trial. The wounds were treated with the PWD with gentamicin cream or SoC dressings. The wounds were evaluated before the start of treatment and after 48-96 hours via clinical assessment, photographs, and qualitative bacterial swabs for bacterial analysis. The delivery of gentamicin via the PWD was safe and did not cause any adverse effects. The treatment decreased both inflammation and bacterial growth during the study period. No significant differences in the SoC were observed. The PWD is a transparent and impermeable polyurethane chamber that encloses and protects the injured area. The delivery of topical gentamicin via the PWD was safe and effective. Clinical assessment for infection found the PWD to be non-inferior to the current SoC treatment options.
Keywords: gentamicin cream; platform wound device; topical treatment; wound healing; wound infection.
© 2022 The Authors. International Wound Journal published by Medicalhelplines.com Inc (3M) and John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Eriksson is the founder and chief medical officer of Applied Tissue Technologies LLC (ATT). He has not participated in the evaluation or recording of the results or the formulation of the conclusions or summary. Dr. Nuutila used to be an employee of the ATT. All the other authors have no conflict of interest to declare in relation to the content of this article.
Figures

References
-
- Bowler PG. The 10(5) bacterial growth guideline: reassessing its clinical relevance in wound healing. Ostomy Wound Manage. 2003;49(1):44‐53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

